Firmament by Clark
Ref: Simon Clark (2022). Firmament. The Hidden Science of Weather, Climate Change, and the Air that Surrounds Us. Hodder & Stoughton.
___________________________________________________________________________
Summary
Starting from the earliest pioneers of atmospheric science, we’ll meet a cast of characters from around the world – not only remarkable scientists, but also physics and phenomena that will open your eyes to how incredible the air we breathe is. We’ll begin by sketching out the anatomy of the atmosphere, introducing you to its layers, each with their own personalities and quirks. We’ll look at the giant’s blood – how and why air and moisture flow from one part of the globe to another, and the fascinating history of this understanding. Certain organs in the giant are particularly interesting, and we’ll look at these in some detail – the great ribbon of air that forms the mid-latitude jet stream, the bulk of the colossal stratospheric polar vortex. Perhaps most mind-boggling of all is the way that the atmosphere exhibits teleconnections, the phenomenon of particular see-saw patterns influencing the giant’s footprints on our lives even from the other side of the world, and we’ll meet these in turn too.
Atmospheric science has a history as long and noble as any other field of research. Like many sciences it can trace its roots back to antiquity, though it is really in the Renaissance and the Early Modern period that it came into its own. It has strong connections with chemistry, physics, and geology, and some of the greatest scientists in history have contributed to our knowledge.
Atmospheric science is essential. The invention of another technology – the steam engine – has forced us to confront a new reality. Our atmosphere is changing, and we are responsible.
Climate change – and its associated uncertainties – has come to dominate the popular discourse around our planet’s atmosphere: changes in the concentrations of CO2 and other trace gases causing increases in temperature, rising sea levels, retreating glaciers, and more extreme storms.
What is likely to happen by 2100? A summary that scientists can be reasonably confident in is this: carbon dioxide concentrations will likely peak between 500 and 600 ppm. This will lead to a global warming of around 2 °C. Changes that are expected to take place as a result of this include: average sea levels rising by 2m, CAT 5 hurricanes doubling in frequency, several billion people losing ready access to fresh water, tropical diseases becoming far more common further from the equator, an increase in migration and resource-based conflicts, and a mass extinction of plant and animal species occurring on a scale that has not been seen while humans have existed on Earth.
___________________________________________________________________________
Intro Giant
___________________________________________________________________________
Ch 1 Idea
The concepts of science and scientist didn’t exist until the 19c. Before then, the terms natural philosophy and natural philosopher were used instead to refer to the study of nature and to someone who studied it, respectively.
The first natural philosopher is considered to be Thales of Miletus (624–545 BCE), who, as well as being credited with inventing the concept of a mathematical theorem and associated proof, was intensely interested in weather…Thales travelled the ancient Med extensively and was said to have visited Egypt to witness the annual flooding of the Nile. While the Egyptians explained the flooding as being the result of the god Hapi’s arrival, controlled by the pharaoh, Thales gave a different, natural explanation. According to his theory, northerly winds prevented the Nile from flooding most of the year by pushing the river water upstream. In flooding season, however, this wind disappeared and so the river would burst out, unopposed, into the floodplain.
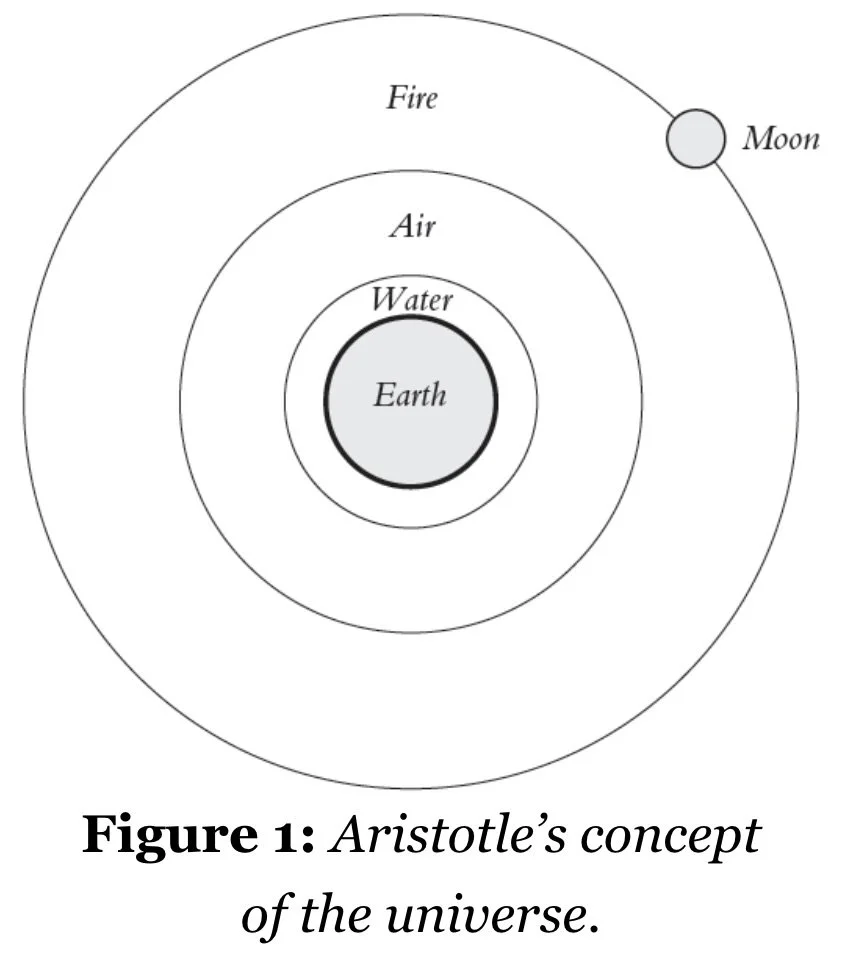
Aristotle borrowed from Eudoxus of Cnidus (c.390–c.337 BCE) the idea that the universe was divided into concentric spheres with the Earth at its centre. Around the Earth was a terrestrial sphere bounded by the orbit of the Moon. Above this terrestrial region was the celestial sphere…This division, Aristotle argued, necessitated separate fields of science for each region: astronomy for the celestial sphere, and his new field, meteorology, for the terrestrial sphere beneath. This sphere, extending to the Moon, consisted of four elements – earth, water, air, and fire – as adapted from Empedocles (c.494–c.434 BCE), arranged in concentric layers. Earth lay at the bottom with water on top, which was then topped with air, which in turn was topped by fire.
At the end of the 13c, a series of innovations are made by Venetian glass makers; experimenting with new compounds added to the silica that forms the body of glass, successive generations of glassblowers were able to make their products stronger and more malleable. They realized that by adding the burned ash of certain plants, their glass could be made perfectly clear, and far more resistant to attack by chemicals or sudden changes in temperature. This made the development of specialist scientific instruments possible – scientists could now isolate chemicals in their laboratories, or construct elaborate devices such as air pumps
In trying to improve Galileo’s (thermoscope) design, Torricelli filled a metre-long glass tube with Hg and carefully upended the tube into a basin, also filled with Hg. The level of metal in the tube, now vertical, fell gradually before stabilising one-quarter of the way down the length of the tube. However, after leaving his equipment for a few days, Torricelli noted that this level started to change. Some days the Hg in the tube fell rapidly, while on others it would slowly climb. Torricelli claimed that, with his changes to the instrument’s design, these changes were caused by variations in atmospheric pressure, the weight of air overhead pressing down on the Earth’s surface, rather than by changes in the temperature.
“We live submerged at the bottom of an ocean of the element air, which by unquestioned experiments is known to have weight.”-Torricelli.
Republic of Letters: An international organization of thinkers that formed in the 16c and allowed for the rapid transmission of revolutionary ideas.
It was well known by mountaineers such as Perier that the higher one climbed, the colder the surrounding air became: the summit of the Puy de Dôme was significantly colder than its base. It was a reasonable extrapolation to believe that as the atmosphere thinned with altitude, it would also become colder and colder. The Earth therefore must possess an atmosphere that was thick and warm close to the surface, and became thin and cold at great altitudes. This suggested the existence of some vast, freezing vacuum between the planets…In 1648, Pascal invented his own barometer and repeated Torricelli’s experiment, coming to the same conclusion: that air had weight and exerted a pressure on the Hg in the basin. He further concluded that if air has weight, then the pressure exerted by the atmosphere should decrease as one climbed to higher altitudes, where the quantity – and therefore weight – of the atmosphere above was lower. Conveniently enough, Pascal had a brother-in-law, Florin Perier, who lived near a mountain – the Puy de Dôme in central France. To test his hypothesis, Pascal tasked Perier with taking measurements with a barometer while climbing the mountain. Perier dutifully carried out this experiment and reported back to Pascal in a delightful letter that, indeed, the Hg in his tube fell lower and lower as he climbed to the mountain’s peak.
___________________________________________________________________________
Ch 2 Birth
Earth’s first atmosphere was mostly H clinging to the surface of the planet…As the Earth cooled and took shape, this H was gradually lost to space, and the planet was left exposed to the void…Over several billion years, volcanic activity and the meteor impacts that were common on the young Earth spewed gases such as CO2, sulphides, and N into the sky. Then, as now, the majority of the atmosphere was N in diatomic form – N2…So, for the first billion years or so in the Earth’s life, then, the atmosphere consisted of N mixed with smaller quantities of water vapour, CO2, and other trace elements.
The earliest life forms were archaea, single-celled organisms distinct from bacteria, plants, or animals. The ancestors of plants were not far behind archaea, however, and within a hundred million years of the first fossils, life had developed the ability to photosynthesize.
Over the next billion years, life produced so much O that the atmosphere started to change in composition. The oceans, previously anoxic, were flush with O. This spelled disaster for the archaea, which had evolved to cope with an O-free environment, and a mass extinction ravaged the planet in what is now called a variety of names, from the Great Oxidation Event to the O Holocaust…Certain organisms, in their attempt to survive the toxic, high-O environment, clumped together to produce a safe haven for their genetic material. Cells with nuclei and organelles like mitochondria were born.
By about half a billion years ago, the atmosphere was recognizably much as it is today – overwhelmingly N with a healthy dollop of O, and a few trace gases such as water vapour, CO2, argon, and several others.
Global temperatures in the Cambrian Period (541–485 Ma) were as much as 14 °C higher than the present day, while temperatures in the Permian Period (299 – 252 Ma) were maybe 3 °C colder than now.
The colder a planet is, the more ‘light’ water is trapped in the polar regions as ice, and the less O-16 there is to go around – and so, the larger the overall ratio between O-18 and O-16 found in the warmer water areas. Or, in other words, the ratio of O isotopes found in water molecules close to the equator can tell you roughly what the Earth’s average temperature is…More O-18 than expected? The planet is cold (the O-16 is locked in polar ice). Less O-18? The planet is warm (there is little polar ice).
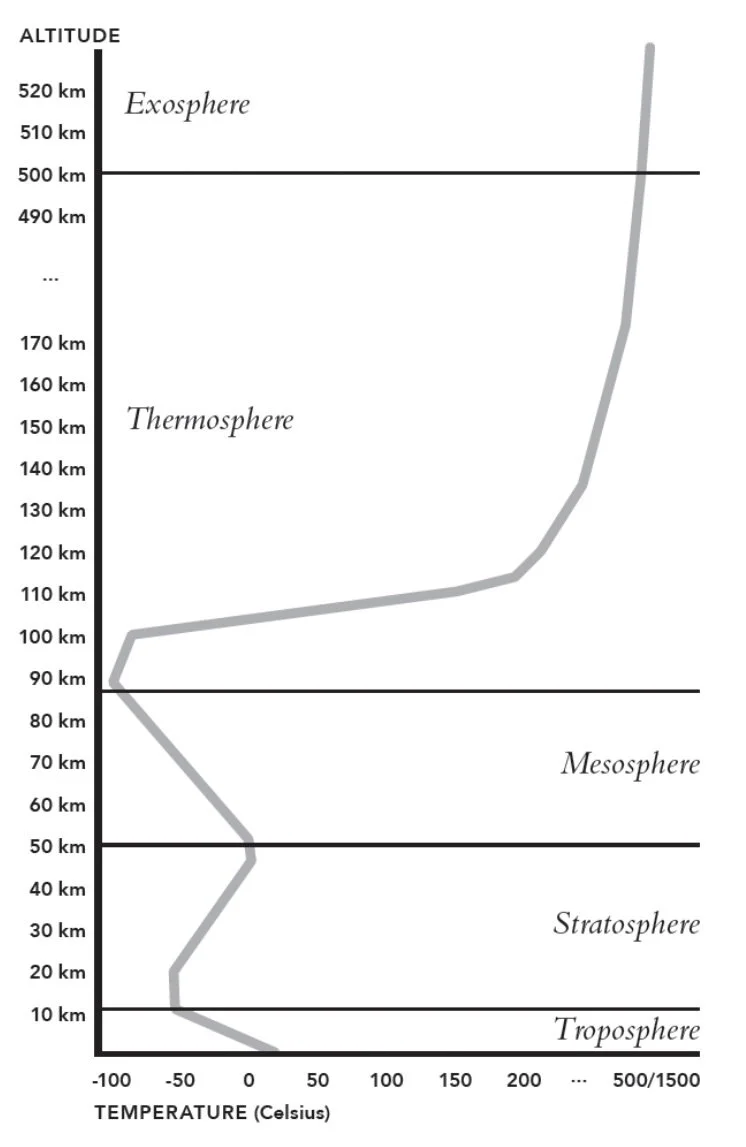
Stratosphere: The second lowest layer of the Earth’s atmosphere, characterized by increasing temperatures from a low at ~10km to a high at roughly 50km in altitude; discovered by Assmann, who called it a permanent temperature inversion zone and Teisserenc de Bort, who called it an ‘isothermal layer’.
Mesosphere: From ‘meso’- middle; an atmospheric layer from 50- 80km in altitude where air temperature stalls and remains approximately constant for a few kilometres before decreasing with altitude.
Thermosphere: from ‘thermo’- heat; An atmospheric layer above the mesosphere from 80km upwards, in which the air temperature increases with altitude. Notable as the location of the aurora borealis and australis.
Higher reaches of the atmosphere absorb high-frequency solar radiation, and as a result could be ionized. In the process of absorbing high-energy UV and X-ray radiation, gas in the upper atmosphere is ionized and greatly heated.
___________________________________________________________________________
Ch 3 Wind
At the end of the 13c, a series of innovations are made by Venetian glass makers; experimenting with new compounds added to the silica that forms the body of glass, successive generations of glassblowers were able to make their products stronger and more malleable. They realized that by adding the burned ash of certain plants, their glass could be made perfectly clear, and far more resistant to attack by chemicals or sudden changes in temperature. This made the development of specialist scientific instruments possible – scientists could now isolate chemicals in their laboratories, or construct elaborate devices such as air pumps
Defoe surmised that several reports were all related to the same 1703 storm, one that had come from the west, passed over England, France, and Germany, and then continued out over the Baltic Sea, leaving destruction in its wake. This was no in situ weather event! Wind had carried the same atmospheric conditions over a large area.
1743, American polymath Benjamin Franklin (1706-90) was attempting to observe a lunar eclipse in Philadelphia, but was thwarted by clouds. His correspondents in Boston reported, however, that their view of the eclipse was good, with clouds only arriving an hour after the event ended. Franklin correctly realized that the clouds he was unfortunate enough to be under were the same as those later seen by his Bostonian friends.
1821, Redfield was out walking when he noticed that trees blown down by a storm had fallen in a spiral. This, he reasoned, must have been caused by the pattern of winds in the storm. Redfield went on to conduct an analysis of other storms that had similarly blown up the east coast of the US from the Caribbean. He found that these storms all shared a similar pattern of winds, a pattern he described in a paper in 1831 as a ‘progressive whirlwind’ with a calm centre, rotating anticlockwise. Today we would call this pattern a cyclone (kúklos- ‘circle’ in Greek), a rotation of the fluid of the atmosphere around a point.
1858, Ferrel publishes ‘The Influence of the Earth’s Rotation Upon the Relative Motion of Bodies Near its Surface’. This tiny paper, just four pages long, is a true milestone in meteorology, crammed with elegant mathematics that seem to burst out of the margins. It was the first time that someone had combined all the pieces necessary to predict how air behaves on the surface of the Earth. Using data collated by mathematician and fellow meteorologist James Henry Coffin (1806–73), it combined Newton’s second law (that the force acting on an object is equal to its mass multiplied by the acceleration it experiences, or F=mA) with rotational mechanics necessary to calculate the deflection a parcel of air experiences as it moves around a rotating sphere. Ferrel the farm-boy had put numbers to the flow of the atmosphere, and in so doing explained Redfield’s ‘Circular Theory’: why air rotates around the low-pressure heart of a storm. Air rushes to occupy an area of low atmospheric pressure, yes, but in doing this it is deflected by the rotation of the Earth. This is known as the Coriolis deflection.
___________________________________________________________________________
Ch 4 Fields
Wind is forced by air pressure, which is in turn forced by air temperature.
The Earth occupies a mere fifty billionths of 1% of space visible to the Sun, and drinks in ~150K trillion J of sunlight every single second.
Every object constantly emits energy in the form of EMR, which we call blackbody radiation. The reason we don’t see all objects glowing like the Sun, however, is that the quantity of blackbody radiation emitted depends on how hot the object is: the hotter the object, the more energy it emits per second. To be a little more specific, the quantity of energy emitted is proportional to the fourth power of an object’s temperature, i.e., T4, where the temperature is measured in kelvin…The quantity and wavelength of energy emitted by an object is dependent on the temperature of the object…As an object gets hotter and hotter, it emits more and more short wavelength blackbody radiation, and less long wavelength radiation…This is because short wavelengths of light carry more energy than long wavelengths of light, and so, to more efficiently transport heat away from an object, the hotter the object gets, the more it prefers to emit short wavelengths of light…A relatively cool object like the Earth (having a temperature of a few hundred kelvin) doesn’t emit much energy, and does so mostly at long wavelengths…The Earth largely emits wavelengths in the IR part of the spectrum, having wavelengths just a little longer than visible light. The Sun, however, being extremely large and extremely hot (thousands of kelvin at its surface), pumps out huge amounts of energy, largely at shorter wavelengths such as UV light.
Sunlight – mostly short wavelengths of light, fresh from the stellar forge – passes through the Earth’s atmosphere and is absorbed by the planet underneath. The Earth, drinking in this energy, heats up and then, like all objects in the universe, emits blackbody radiation of its own. Because it is relatively cool, the Earth mostly emits longer, IR wavelengths of light upwards, into the atmosphere. However, while the atmosphere is perfectly happy letting short wavelength sunlight pass through it, it’s nowhere near as lenient when it comes to earthlight and its IR radiation. In fact, our atmosphere appears like a brick wall to long wavelengths of light, and absorbs pretty much all of the energy the Earth emits as blackbody radiation. Having done this, the atmosphere heats up and then, of course, emits blackbody radiation of its own – half of it outwards, into space, and half of it inwards, towards the Earth…The notable exception to this is of course the absorption of short wavelengths of light by ozone.
The atmosphere is not heated directly from above by the Sun, but instead heated from below by the Earth.
Air at the surface is heated by the Earth below, but this heating isn’t uniform – some areas will see higher air temperatures than others, such as air over land compared to air over water. Where air is warmer than its surroundings, it is also less dense. We know this because our equation of state says the pressure air experiences is proportional to its density multiplied by its temperature. As air is under pretty much the same pressure everywhere at the surface – pressure being nothing more than the weight of air above pressing down – this means that if the air temperature increases, air density must decrease in order to balance our equation of state. A bundle of less dense, warm air surrounded by more dense, cool air is then in much the same situation as a bubble of steam at the bottom of a pan of water on the stove – it rises! We call this process convection (‘to carry together’- Latin), and across the Earth we see vast overturning circulations driven by convection.
___________________________________________________________________________
Ch 5 Trade
The flow of the atmosphere is ultimately caused by changes in air pressure…Air pressure is in turn forced by temperature; specifically, the temperature of the planet beneath it.
in the N. hemisphere’s summer, the arid deserts and steppes of western Asia scorch in the sun. Daytime temperatures can regularly soar over 40 °C, and long days, strong sunshine, and little moisture bring extreme heat to Iraq, Turkey, Syria, and Iran. As you may have guessed, this causes convection – air pressure has remained constant but air temperature increased, and so the air density decreases according to the equation of state. Being less dense than the surrounding air, the warmer air is more buoyant, and so rises into the sky. This leaves behind a partial vacuum, and so the air pressure near the surface falls. As a result of this, a system of low pressure known as the Asiatic or Iranian low dominates western Asia in the summer months. Combined with the relatively high pressure in central Europe in summer (fueled by a much moister climate), air is funneled between the two systems in what is known as an atmospheric trough. Every year, this causes air to flow from the warm, mostly dry interior of eastern Europe towards the Mediterranean. This air flow is what we call the Etesian wind, blowing year after year, keeping Athens’ fleet at harbour.
1686: Halley pens ‘An Historical Account of the Trade Winds, and Monsoons, Observable in the Seas between and Near the Tropicks, with an Attempt to Assign the Phisical Cause of the Said Winds’; arguably the first academic paper ever written in the modern field of climatology.
“I could think of no better way to design the course of the winds on the mapp, than by drawing rows of stroaks in the same line that a ship would move going alwaies [always] before it; the sharp end of each little stroak pointing out that part of the Horizon, from whence the wind continually comes.”-Halley, 1686.
15c: Portuguese sailors first recognize the ability of ships to sail from Europe to North America by the trade winds in the Atlantic Ocean.
1735: Shortly after being elected a Fellow of the Royal Society, Hadley publishes a paper on the subject, ‘Concerning the Cause of the General Trade Winds’. He improves on Halley’s theory by first, dismissing the idea that warm air rushed in from behind the path of the Sun (if this were the case then the whole planet would experience persistent easterly winds). Instead, Hadley asserted that when air was heated in the tropics and ascended, it was replaced by air not from the E., but from both N. and S. of the equator, where the heating by the Sun was marginally less. This air rushed towards the equator, where it converged. Hadley’s second improvement was that the rotation of the Earth would affect these converging air masses.
An air parcel that moves northward from the equator is deflected to the right, i.e., eastward.
1835: Coriolis publishes ‘On the Equations of Relative Movement of Systems of Bodies’ (in which he does not reference the atmosphere or the rotation of the Earth)- examining the transfer of energy in general rotating systems, using waterwheels as an example.
___________________________________________________________________________
Ch 6 Distance
___________________________________________________________________________
Ch 7 Forecast
1890: American meteorologist Cleveland Abbe (1838–1916) proposes that meteorology is simply the application of the laws of physics involving fluids and thermodynamics to the atmosphere.
1904: Norwegian meteorologist Vilhelm Bjerknes (1862–1951) argues that weather forecasting should be possible using mathematical methods. Bjerknes basically argued that you needed to follow four steps to predict what the atmosphere will do next: 1 Take measurements of atmospheric fields such as pressure and temperature, providing you with the initial state of the atmosphere. 2 Calculate expected changes in the velocity fields using your observed initial state and the primitive equations. 3 Using the updated velocity fields, calculated in the previous step, work out the expected changes of fields such as temperature and humidity due to advection. 4 Repeat steps 2–4. His ideas were so influential that they evolved into the Bergen School of Meteorology, a school of thought used in basically all of modern atmospheric physics, and particularly adopted by influential early 20c Scandinavian scientists such as Carl-Gustaf Rossby. These scientists used the rapidly developing observation networks, considerably expanded during the two World Wars, to construct what we would now recognize as modern meteorology.
Weather Fronts: Named due to their resemblance to battle lines on the western front of WWI.
Cold Front: Occurs when a cold air mass rolls over the landscape, displacing and uplifting warmer air. The uplift can produce sharp changes in weather, such as rapid cloud formation and heavy precipitation. Denoted by triangles.
Warm Front: Denoted by semicircles.
Advection: The process of a fluid carrying stuff to places.
Interacting air masses behave as fluids.
Numerical Weather Prediction: Using the equations of the Bergen School to predict the future state of the atmosphere given current measurements – by computers allows for quantitative prediction.
A computer model used for quantitative weather prediction stores the information describing an atmospheric field, such as temperature or humidity, in a grid. The grid size or resolution determines how many data points are used to describe the field – if we are considering a field on a global scale, a resolution of 2.5° might be used, indicating that a value is stored at every 2.5° in longitude and latitude. The higher the resolution, the more accurately the continuous field is described, though this makes computation slower.
The UK Met Office uses a resolution of 1500m when running forecasts over the British Isles.
Deterministic Nonperiodic Flow (‘Chaos Theory’): Examines dynamical systems that may appear to behave randomly but are in fact governed by deterministic equations, like Newton’s laws of motion. These equations are combined in such a way that outcomes are extremely sensitive to initial conditions. Lorenz neatly summarized it as: ‘Chaos: when the present determines the future, but the approximate present does not approximately determine the future.’
Weather is governed by a small set of equations with definite outcomes, just ones that are very sensitive to the initial conditions put into them.
Ensemble Forecasts: Predicting the weather several times, but initializing each set of calculations with a slightly different set of initial conditions. One prediction may take the upper end of the uncertainty in the temperature field as its initial condition, for example, while another may take the lower end of the uncertainty in the temperature field as its initial condition. Each of these forecasts will produce different end results, which may be very similar, or wildly different. Taking the average of all these forecasts produces one, more statistically robust, forecast.
The inherently chaotic nature of the atmosphere means that even with the most widespread measurements possible, the highest-resolution computer model, and the most accurate instruments, the small error bars present in the initial conditions of the atmosphere eventually propagate to huge errors in forecasts far into the future. Practically, this means that science can predict the weather really quite accurately for about ten days into the future before the errors become unacceptably large.
___________________________________________________________________________
Ch 8 Vortex
The static stability of the stratosphere inhibits almost all vertical motion, causing air to flow horizontally instead, as if on a flat surface, in far larger patterns. With no vertical motion to disrupt them, immense atmospheric structures the size of continents can form. Instead of splintering into small storms, these titanic circulations grow larger and stronger over the course of the year. The only thing that checks their growth is the changing of the seasons.
From the ideal gas law, a decrease in temperature with no change in air density will result in a decrease in atmospheric pressure. And when areas of low atmospheric pressure form on the spinning Earth, air will rotate around them in a cyclonic circulation. A vast, hemisphere-scale circulation therefore develops around the polar region in winter, covering an area the size of Asia. When summer arrives, the Sun rises on the polar region once more, and a huge amount of energy in the form of sunlight is added. Thanks to the absorption of UV radiation by ozone, this causes a spectacular rise in temperature, and the summertime polar stratosphere is warmer than the equatorial stratosphere. This destroys the wintertime circulation, and the polar regions are now home to higher air pressure than the low latitudes. As such, an anticyclonic system, spinning in the opposite direction, forms.
An increasing pressure gradient with altitude produces a wind shear, as the strength of the wind is proportional to the strength of the pressure gradient, and as the gradient gets steeper with altitude, the strength of the wind (the thermal wind) also increases…As winter sets in across a hemisphere, however, causing the Sun to set for months at a time, temperatures plummet, and a temperature gradient is established in the opposite direction: now the polar air is significantly colder than air at the equator…The thermal wind effect causes this circulation to be much, much stronger in the stratosphere than in the troposphere, accelerating the wind to breakneck speeds. The huge rotating circulation that results is the stratospheric polar vortex, the ghostly titan of the atmosphere. This is the culprit ultimately behind the frozen outbreaks of air in London and Chicago. A polar vortex forms every year in whichever hemisphere is currently experiencing winter, stretching from approximately 15 km to more than 50 km above the surface. The stratospheric polar vortex is vast. The largest tropical storm ever observed, Typhoon Tip, was over 2,000 km across and had wind speeds peaking at 300 kph (190 mph). By contrast, the polar vortex is around 6,000 km from edge to edge
Sudden Stratospheric Warming (SSW): Occur when air temperature in the middle atmosphere increase by as much as 60 °C in just a few days- affecting the entire region of the stratospheric polar vortex. SSWs are observed all over the Arctic sky, occurring on average six times every decade…When an SSW occurs, the polar vortex – the continent-sized doughnut of air spinning at 200 kph – tears itself apart. The immense warming fundamentally destabilizes the hemispheric temperature gradient that drove the vortex, resulting in its destruction. Sometimes this involves the circulation making a sudden journey equatorward, eventually hitting such warm air that it cannot sustain itself, while sometimes the circulation stays in place but tears itself into two smaller vortices. These two circulations then fight it out, always resulting in the total destruction of both. Either way, a rapidly spinning mass of air the size of Asia, over the course of perhaps a week, utterly destroys itself.
The vortex forms as winter sets in across a hemisphere. In the N. hemisphere this is typically around November. Through the winter the vortex maintains a strong westerly circulation, but as spring arrives sunlight creeps into the polar regions and causes them to warm, and the vortex gradually gets weaker and weaker. The stratosphere’s transition from wintertime regime to summertime regime is not as smooth, however. While in late autumn the vortex enters the scene slowly without much of a fuss, in spring it exits stage with a bang. Or, more precisely, an explosive warming. The vortex shreds itself in what is known as a final warming. This final warming occurs every year, and marks the end of the winter season. SSWs on the other hand can occur at any time in the winter, and are followed by the vortex pulling itself back together and resuming a westerly circulation over the course of a few weeks, before eventually succumbing to a final warming in spring…The southern polar vortex is much stronger and more uniform than the northern polar vortex. In all our years of observations, the southern vortex has only experienced three SSWs (and one of those was very minor).
Different temperatures – such as over land and over oceans – along lines of constant latitude produce areas of high and low pressure, which we call atmospheric waves. These waves can move, or, more properly, propagate, both over the surface of the Earth and also vertically up or down through the atmosphere. We see this as areas of high and low pressure moving in tandem around a line of constant latitude, much as we see ocean waves moving together as peaks and troughs in the water…Atmospheric waves are described by the same fluid dynamics as ocean waves and can therefore also ‘break’ in the same way when they encounter a region through which they can no longer propagate. However, for atmospheric waves, this takes place where certain atmospheric conditions are no longer met. Specifically, this occurs at the point where they encounter the stratosphere and meet the polar vortex, in a region just outside the edge of the vortex called the stratospheric surf zone. Here, atmospheric waves ‘break’ – or deposit their momentum, to describe it a little more formally – but instead of eroding the landscape, this deposition of momentum acts to slow the stratospheric polar vortex down.
The polar vortex is like a free-spinning bike wheel on its side, constantly accelerated by the equator-to-pole temperature gradient, the breaking of atmospheric waves acts as a brake on the wheel, preventing it from accelerating to ever greater speeds. The stronger the braking force, the slower the wheel ends up spinning. As the northern hemisphere has a large amount of both land and sea – it is highly zonally asymmetric – it produces strong atmospheric waves, which act as a significant brake on the northern polar vortex when they encounter the stratosphere and deposit their momentum. By contrast, the southern hemisphere is largely zonally symmetric and so produces weaker atmospheric waves that act as a weaker brake on the southern vortex when they break in the southern hemisphere stratosphere. As a result, the southern vortex is much stronger than the northern vortex, even though they are both accelerated by the same temperature gradient…When the wave activity is particularly large, the brake on the vortex can become so powerful that the wheel stops spinning entirely, and the polar vortex careens to a halt. As the northern hemisphere has a higher-than-average wave activity, when the additional factors like local precipitation are stronger than normal, this results in enough wave forcing to cause a sudden stratospheric warming. Compare this to the southern vortex, where the average wave forcing is lower, and the additional factors would have to combine to their absolute maximum to produce enough wave forcing to disrupt the vortex…Effectively, the excess wave breaking brings the flow of the upper vortex down to a speed at which atmospheric waves can no longer propagate, forming a ‘critical surface’. This critical surface then sees additional wave breaking, which lowers the surface in altitude. This process repeats until the whole vortex has been destroyed, and waves can no longer propagate in the stratosphere.
1999: Scientists discover that when the N. hemisphere polar vortex breaks apart in an SSW event, the jet stream is diverted.
Storms in the Atlantic tracked further south in the aftermath of an SSW. Subsequent work by the team also identified that cold weather became more extreme in the mid-latitudes, growing more likely and more intense. Both of these phenomena were a result of the wayward jet stream. The areas of low pressure formed by waves in the jet stream diverted storms at lower latitudes, bringing strong winds and intense precipitation in areas that otherwise wouldn’t experience them. Equally, these meanders in the jet stream allowed frigid Arctic air to spill further south. This is exactly what happened in 2018 and 2019 – the British Isles and the Great Lakes were caught under a southward meander of the jet stream and froze under the freshly unchained Arctic air. Especially when combined with a source of moisture, such as an Atlantic gale, this is a perfect storm for wintertime chaos.
There are several ‘strategic points’ of world weather. The Southern Oscillation in the Pacific is one such strategic point, influencing the Indian monsoon and weather patterns all over the world. Another strategic point is located in the North Atlantic, where scientists find another see-saw of atmospheric pressure, this time roughly between Iceland and the Azores. This came to be known as the North Atlantic Oscillation or NAO, and just like the Southern Oscillation it could also be described with a numerical index. This NAO index broadly represents the influence of the jet stream on the weather in Europe. When the NAO index is high, the jet stream tracks further north than usual, and Europe experiences more fair weather, while if the NAO index is low, the jet stream tracks further south, and Europe experiences colder, stormier conditions.
With the influence of an SSW typically taking a few weeks to propagate to the surface, meteorologists can observe an SSW taking place in the stratosphere and be on the lookout for tell-tale signs of trouble in the troposphere in the weeks ahead…Some models see a greater improvement in forecast accuracy by including the stratosphere than by improving their spatial resolution.
___________________________________________________________________________
Ch 9 Change
Scottish gentleman scholar James Hutton (1726–97), referred to by many as the father of modern geology, proposed that erratics were caused by glacial retreat – the boulders had, in the past, been enclosed by ice, which then flowed downhill, carrying the rocks away from their native geologies. The ‘mouth’ of the glacier – the ablation zone – experiences loss of ice due to melting, calving, and other processes, and eventually the boulders would tumble out of the glacier when the surrounding ice melted. However, many erratics were found at great distances from the nearest glacier. It is unclear if Hutton had an explanation for this, but subsequent authors proposed a radical one: glaciers had previously covered a much greater part of Europe, and the climate was at this time much, much colder. The Swiss-born Louis Agassiz (1807–73) theorized that the Earth had previously experienced Die Eiszeit or an Ice Age, a cold period in the planet’s history during which Alpine glaciers extended to cover much of Europe. In fact, Agassiz claimed, almost the entire N. hemisphere, including every N. continent, had been covered with ice sheets.
Orbital Eccentricity: The ellipse of a planet’s orbit; a planet with an eccentricity of 0 has a perfectly circular orbit (Venus has an eccentricity of .007), while a planet with an eccentricity of 1 has a parabolic orbit.
The Earth currently has an almost circular orbit, with an eccentricity of just 0.017. Croll argued that while this eccentricity was small, it could be significant enough to alter the Earth’s climate. When the Earth is slightly further away from the Sun in its orbit, it receives less solar radiation, and so less heating. Equally, when its orbit brings it closer to the Sun, the planet receives more solar radiation and so more heating. In itself this should not produce a significant change in the Earth’s climate – after all, the two different contributions should cancel out over the course of a year. However, the times of year that the orbit is close and far from the Sun slowly vary on an astronomical cycle – tens of thousands of years long – known as apsidal precession. Croll argued that there was an important feedback loop taking place. If the winter of a hemisphere coincided with the section of the orbit where the Earth was far from the Sun, the resulting extreme cold would cause extensive snowfall and ice formation. This would blanket the surface of the winter hemisphere in white, making the surface more reflective. Scientists describe this as increasing the albedo (‘white’- Latin) of the surface. This more reflective surface would then absorb less solar radiation, leading to deeper cold, and even more ice formation. If the resulting cold was extreme enough, ice could persist into the summer months, leading to year-round wintery conditions: an ice age. Eventually, the precession of the Earth’s orbit would lead to warmer conditions in winter, and the ice age would give way to a warmer period. Croll’s ice albedo feedback theory thus predicted alternating periods in the Earth’s history – ice ages with ice-covered poles followed by interglacials with ice-free summers…It would take a further scientist, the brilliant Serbian/Croatian Milutin Milankovic´ (1879–1958) to add the necessary extra complexity to Croll’s theories. Milankovic´ crucially corrected a mistake in Croll’s argument - winters coinciding with a hemisphere being furthest in the Earth’s orbit from the Sun was not the cause of the ice ages. Instead, it was when summers coincided with this furthest point in the orbit, reducing how much snow and ice thawed before the following winter. This reduced melt combined with the same quantity of snowfall in winter caused ice sheets to gradually accumulate. With this, and by accounting for additional elements in the complicated gravitational interactions between planets, accurately predicting changes in the Earth’s eccentricity, axial tilt, and precession, using thousands of painstaking pencil-and-paper calculations, Milanković was able to explain observed ice ages in the historical record.
During an appointment in Grenoble, Fourier began experimenting with the propagation of heat. This was informed by his time in Egypt, where Fourier had decided that heat had life-giving. Using some innovative math’s, Fourier was able to describe how temperature in an object evolves both in time and in space. The math’s he developed, which now bears his name, had immense implications for the rest of science, but so too did the understanding of heat that his methods afforded. Of particular relevance to our story, Fourier, in the last few years of his life, concerned himself with the line of thinking we have been pursuing in this chapter, and found that the Earth should be a lot colder!
Working backwards from the blackbody radiation equation allows us to calculate the average temperature of the Earth if the only processes going on are absorption of solar radiation, and emission of thermal radiation by the Earth’s surface. Doing this, we find a global average temperature of around -18 °C. Compare this to the global average temperature we observe, which is around 15 °C. The planet is over 30 °C warmer than basic thermodynamics says it should be! Fourier concluded that the atmosphere acted like an insulator, somehow providing the extra heating necessary to keep the Earth at habitable temperatures.
Fourier was killed by his obsession with heat. As an old man he kept his house overheated, and even wore excessively warm clothes to try to maximize the imagined healing power of heat. On 4 May 1830, possibly because he was faint after spending a day as a walking sauna, or simply due to tripping on his sweat-soaked dressing gown, Fourier fell down the stairs. He was badly injured, and died just a few days later.
Light can be defined by its wavelength, and different substances will absorb different wavelengths. Ozone, for example, will absorb UV radiation, while oxygen and nitrogen will not.
We can describe substances as being opaque or transparent to certain wavelengths of light – normally, of course, we’d use these words to reference whether an object absorbs visible light. A brick is opaque, while water is transparent, but only to visible light. However, we could just as validly discuss whether these objects are opaque or transparent to, say, UV light. Ozone, for example, is opaque to UV radiation, while N is transparent.
1856: Professor Joseph Henry (1797-1878) presents a two-page paper written by Eunice Foote (1819-1888) at the Eighth Annual Meeting of the American Association for the Advancement of Science in Albany. This paper was years ahead of its time, detailing lab experiments that found certain gases were in fact opaque to IR radiation, and that absorption of this radiation by these gases significantly increased their temperature. The gas that was found to produce the greatest warming was carbonic acid (CO2).
While it is transparent to most of the light radiated by the Sun (mostly short wavelengths, the Sun being such a hot object), the atmosphere is a very effective absorber of the radiation given off by the Earth’s surface (mostly long wavelengths, it being a cooler object). This absorption, as shown by Foote, is done partly by CO2, but mostly by water vapour. At any given time, the atmosphere contains over a thousand billion tons of water, both visibly in clouds and invisibly as water vapour. This water is a very effective absorber of longer wavelengths of radiation, and contributes the dominant part of the atmosphere’s insulating properties. Energy enters the Earth system from the Sun, mostly passing through the atmosphere, is re-emitted as thermal radiation by the Earth’s surface and then prevented from leaving by water in the atmosphere, along with other trace compounds such as carbon dioxide and methane. This acts to keep the Earth much warmer than it would otherwise be, the disparity that Fourier initially calculated. This effect is what is commonly known as the greenhouse effect.
Generally, the Earth’s surface experiences a day-to-night (or diurnal) variation in temperature of around 10 °C.
1909-1910: John Poynting (1852-1914) and Frank Very (1852-1927) coin the term “greenhouse effect” in a series of feuding papers.
1901: Swedish meteorologist Nils Ekholm (1848–1923) publishes a paper explaining the insulating properties of the atmosphere. In this he had used the term ‘greenhouse’ to make an analogy: the atmosphere prevented heat loss into space in much the same way that the glass of a greenhouse does, except that the glass of a greenhouse prevents heat loss by keeping air trapped in one place, stopping convection transporting warm air – and so thermal energy – away. The atmosphere, on the other hand, prevents energy leaving the planet in the form of thermal radiation. The end result is the same, but the mechanism is rather different.
This follows from Fourier’s argument – the more insulating material you put in the atmosphere, the greater the amount of heat that is trapped, and so the higher the temperature…However, the reverse is also true: when the temperature increases, so too does the concentration of CO2. This muddies the waters somewhat. This has led some people to suggest that changes in CO2 lag behind changes in temperature, which is only partially correct. In the case of the past several hundred thousand years, the observed changes in temperature have been largely due to changes in the Earth’s orbit – Milanković cycles. When the planet comes out of an ice age, the amount of energy it receives from the Sun increases, melting ice and warming the oceans. The process of doing this causes the oceans to release CO2 into the atmosphere, which then goes on to cause additional warming.
The ocean releases CO2 when warmed for chemical reasons – the warmer water is, the less CO2 can be stored in it. You can test this for yourself by taking two cans of carbonated drink, keeping one in a fridge and the other at room temperature. After a few hours, open both the cans. The can in the fridge will hiss when opened due to the CO2 previously kept bound up in the drink escaping. The can that was kept at room temperature will hiss much more loudly however, as the water in the drink will have been unable to retain all the CO2 it originally contained, and have outgassed it to the air pocket inside the can. When the can is opened, this extra gas rushes out, causing a louder hiss.
It’s estimated that ~90% of the total warming experienced when Earth comes out of an ice age occurs after the increase in CO2 given off by the oceans, so while it is true that some CO2 increase does lag behind increases in temperature, the vast majority of warming follows an increase in atmospheric CO2. In the recent past, this relationship has been muddied by variations in the Earth’s orbit dipping the planet into and out of ice ages. In the more distant past, when the planet was much warmer and hence not experiencing regular glacial/interglacial periods, the principal driver of climate was the varying concentration of CO2 in the atmosphere.
Why did atmospheric CO2 vary so much during the Earth’s history? We’re talking about truly enormous changes, so you would expect the cause to be similarly epic. The truth is actually rather banal. If you’re reading this in the UK, the answer probably lies outside your window: rain. When rain falls through the atmosphere, it picks up very small amounts of CO2 to create a weak carbonic acid. This removes C from the atmosphere, and when the rain runs off into the oceans, it puts the C into deep storage. This storage might be as carbonic acid in the ocean, or in the Earth’s interior as it gets pulled down into the mantle at destructive tectonic plate boundaries. Alternatively, if the rain falls on volcanic rock, it is absorbed straight into the land, while if it lands on carbonate rock it actually slightly dissolves the surface and releases more CO2 into the atmosphere. Eventually, C that is in deep storage will be re-emitted into the atmosphere via volcanic activity and constructive tectonic plate boundaries. In short, there is a geological C cycle that takes place over millions and millions of years. Depending on the configuration of land and sea – whether the continents lie close to the equator or towards the poles, or if areas with carbonate rocks coincide with areas of high precipitation – the balance of C in the atmosphere and in the deep storage of the oceans and crust can alter over long timescales. This internal variability has ultimately caused the concentration of atmospheric CO2 to change, leading to monumental shifts in the Earth’s climate over the past billion years or so.
On long, geological timescales, these changes are due to the global C cycle, and then modified and amplified by other factors such as James Croll’s ice feedback cycle, and the chemistry of C in water. On shorter timescales of tens or hundreds of thousands of years, these changes are due to variations in the Earth’s orbit that are spectacularly amplified by processes internal to the planet. We just so happen to live in a time of relatively little CO2 in the atmosphere, and hence these orbital changes can have enormous effects.
Theophrastus’ On the Causes of Plants might seem an unlikely place to find the first hints of the end of the world. Written at the turn of the 4c BCE, and mostly concerned with types of trees, shrubs, and cereals, Theophrastus notes that in several places in the E. Med where trees have been felled or marshes drained, the local weather was consistently different compared to before these alterations. The air was colder, frost more frequent, and the local wine ruined. In other words, the climate local to these areas had been altered by the economic activity of humans.
In South America, von Humboldt noted that deforestation of parts of the Amazon rainforest in Venezuela was causing a dramatic fall in the level of Lake Valencia. He later wrote: When forests are destroyed, as they are everywhere in America by the European planters, with an imprudent precipitation, the springs are entirely dried up, or become less abundant, the beds of the rivers remaining dry during a part of the year, are converted into torrents, whenever great rains fall on the heights.
360-300 Ma: Origin of coal; an order of plants known as the Lepidodendrales (‘scale tree’- Greek), the earliest tree-like plants with large leaves and thick trunks but relatively shallow roots, dominate Earth. Their wood was made of the polymer lignin which, together with cellulose, gave the plants the ability to stand tall and rigid, with some reaching 50m in height. When Lepidodendrales were falling in Carboniferous forests, there were no bacteria to break down the lignin. Instead, the fallen wood just sat there, untouched, for millennia. Over time, more and more trees would fall on top of one another and compress the earlier trees, first into peat and eventually into coal. The huge quantities of C buried in the ground during this time is from where the Carboniferous Period takes its name – and while coal formation continued for hundreds of millions of years after, it’s estimated that some 90% of the coal we use for fuel today comes from these ‘coal forests’.
The first practical steam engine was instead invented by Thomas Newcomen (1664–1729) an English ironmonger. His invention – the Newcomen engine – was the spark of the Industrial Revolution. His was a huge device that generated steam in a sealed cylinder using a coal-fired boiler. Cold water was then injected into the cylinder, condensing the steam and dramatically decreasing the pressure in the cylinder. The cylinder head, motivated by this partial vacuum from below and atmospheric pressure from above (hence the name atmospheric engine), descended to compress the air in the cylinder. In so doing, the downward motion of the engine generated mechanical force that was used to raise the other end of a beam balanced on a fulcrum. Eventually, the weight of the beam would raise the cylinder head, and steam would be allowed to enter into the cylinder again. This cycle was then repeated ~12x/min, producing the power equivalent to 20 horses. Newcomen’s engine was a success, and was used across England to pump water out of mines. By simply burning a lump of black earth, without the labour of men or horses, mines could be kept dry and productive. Those mines could then be used to produce more metal and more coal, making more money and, crucially, making more steam engines possible.
One day, Watt was asked to repair a small model of a steam engine. Despite popular perception, Watt did not actually invent the steam engine: the first steam engines that modern eyes would recognize were developed earlier, in the 17c, though most were theoretical apparatuses rather than practical ones…When presented by the University of Glasgow with a small model of Newcomen’s engine to repair, Watt realized that he could improve its design. After extensive tinkering, he added a separate chamber where water could condense, keeping it at a constant low pressure. This, and a few other technical additions, spectacularly improved the efficiency of the engine. With the condensation taking place separately from the cylinder, Watt was also able to make the engine push as well as pull. Combining this with his parallel motion (a mechanical linkage that translated up-and-down motion into rotating motion), Watt allowed the steam engine to drive rotary machinery. This would prove to be an earth-shattering accomplishment. Watt’s steam engine, first commercially installed in 1776, at an ironworks near Falkirk, Scotland, could now drive steamships, factories, pumps, and railway engines. British industry now had the ability to convert coal into raw economic productivity. With ample coal supplies available within the country, Great Britain led the charge into the Industrial Revolution.
1873: ~700 people die in London from air pollution.
The air quality was so poor, and the emissions of factories so extreme, however, that some scientists began to wonder just how much coal was being burned by factories around the world.
Swedish geologist Arvid Högbom (1857–1940) compiled estimates of how C cycled through natural processes, such as that emitted by volcanic activity, taken up by oceans, or released by acidic rain, among others. It occurred to him in 1896 to also include man-made C emissions, from factories and railways and so forth. After extensive calculations he was surprised at the result. Human activities were adding roughly as much CO2 to the atmosphere as natural processes already were…He estimated that the total C emitted from burning coal in 1896 would raise the concentration in the atmosphere by only a thousandth part. But if the emissions continued long enough, or if they increased, then perhaps this could be significant.
After a distinguished career in chemistry, Arrhenius found himself interested in Earth’s ice ages. He wondered if he could derive a theory using physical chemistry to explain how the Earth periodically experienced freezing conditions. In particular, he was interested in the role of CO2. He hypothesized that if for some reason the concentration of CO2 in the atmosphere increased – say, after a large volcanic eruption – because of the heat-trapping effect of the molecule, global temperatures would slightly increase as a result. This slight increase would lead to a far more important consequence: the warmer air would hold more moisture. The additional water vapour in the air would then enhance the warming, which could cause more vapour to be taken up into the atmosphere, causing more warming, and so on. Conversely, if the level of CO2 were to decrease, a small amount of cooling would take place, and the air would hold less water vapour. This would cause more significant cooling and, if this feedback loop continued, potentially tip the Earth into an ice age…Simplifying the world down to a few latitude bands, and using data on the absorption of IR radiation by gases that was primitive by today’s standards, Arrhenius spent months arduously calculating using pencil and paper, possibly as a deliberate distraction to his ongoing divorce. Eventually, he published his results. If the concentration of CO2 in the atmosphere – a very minor gas by volume– were to halve, the overall effect on global temperatures would be a cooling of around 5 °C. Conversely, if the concentration of atmospheric CO2 doubled, the Earth would warm by perhaps 5 or 6 °C. Informed by Högbom’s work, Arrhenius predicted that if humanity emitted greater amounts of C into the atmosphere in the future, the global average temperature would gradually increase…Other scientists were skeptical about Arrhenius’s predictions. They pointed out that he had over-simplified the Earth, ignoring myriad complexities, including the effects of clouds in response to changing levels of water vapour. More clouds would surely reflect more light away from the Earth, balancing out the additional insulation provided by water vapour. Additionally, they claimed, it was impossible for CO2 to build up in the atmosphere. If humanity added any of the stuff, it would simply be absorbed into the vast C storage of the ocean, removed from the air…Nature (with a capital N) was eternal, immortal and separate to Man (with a capital M). How could man-made soot possibly affect divine creation? By the turn of the century, most scientists had dismissed Arrhenius as being entirely wrong.
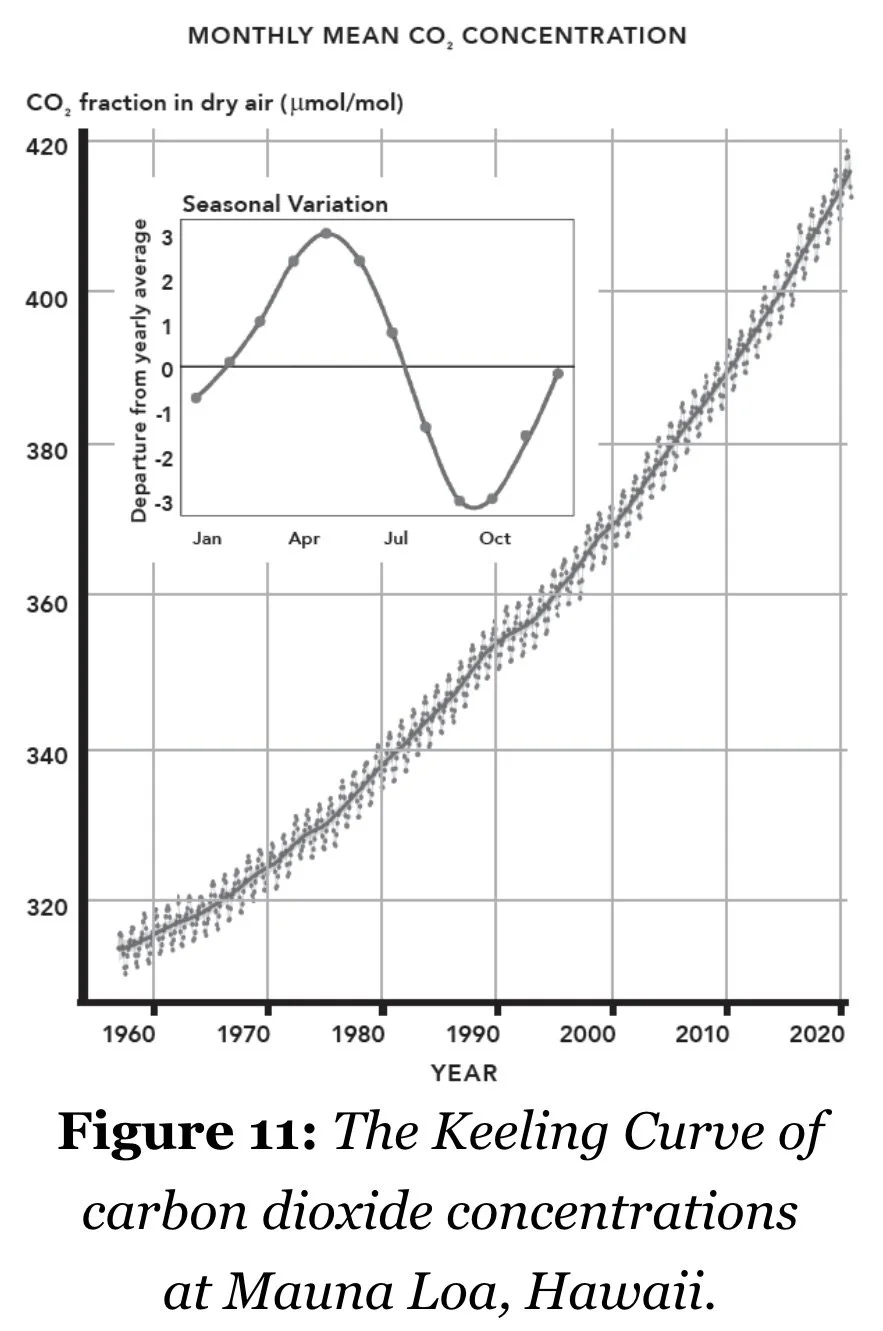
Keeling took air samples every few hours, and from these repeated measurements he identified a number of interesting things. First, CO2 concentrations varied throughout a day – they were lower during the daytime when plants sucked in the gas for photosynthesis, and higher at night when those same plants respired and produced it. Second, CO2 was remarkably consistent. From Maryland to California, CO2 was always between 315 and 320 ppm of the air in his samples…CO2 was well mixed in the atmosphere away from man-made sources such as factories and freeways. This indicated that there was a single value of average CO2 concentrations in the atmosphere. To get an accurate measurement of it, Keeling simply needed to be as far away from human activity as possible. To this end, in March 1958 Keeling began daily measurements of CO2 at an observatory on the north slopes of Mauna Loa, Hawaii…Measurements fluctuated wildly over the course of the year, peaking in May at around 315 ppm and falling to a low of 310 ppm in November. When the concentration began to rise again in December, Keeling realized that he had in fact discovered another cycle of C in the atmosphere. O concentrations in the atmosphere rise and fall with the activity of photosynthesis, and so CO2 concentrations rise and fall in opposition to this. As there is far more landmass, and so far more plants, in the N. hemisphere, this meant CO2 peaked in the N. hemisphere’s summer…Each year should have been easy to predict, a regular sine wave of CO2 concentration across the months…Something was clearly up: the concentration of CO2 in the atmosphere was measurably increasing year on year…For the next four decades he constantly fought to keep the observatory open, scrounging money from different agencies and officials. Except for a hiatus from February to April 1964, the Mauna Loa observatory has kept continuous measurements from 1958 right up to the present day…Prior to the invention of the steam engine, the concentration was estimated to be 280 ppm. In 1958, Keeling measured the concentration to be 315 ppm. By the time of his death – of a heart attack while hiking on his Montana ranch in 2005 – that measurement was 377 ppm, almost 20% more than when he started…Building on the work of Fourier, Foote, Tyndall, and Arrhenius, he had showed that the concentration of CO2 in the atmosphere was increasing, and that, as per Arrhenius’s calculations, this could alter global climate. In 1963 he and a few other scientists published a report ominously titled ‘Implications of Rising Carbon Dioxide Content of the Atmosphere’, detailing that carbon concentrations were increasing by 0.7 ppm per year. If this were to continue for a few centuries – less time if the rate were to increase – then the carbon content of the atmosphere would eventually double, leading to a worldwide warming of up to 3.8 °C…Later that decade, a report by the National Academy of Sciences in the United States indicated that there was no cause for alarm, but that the issue should indeed be closely monitored…Over the next several decades, scientists continued to monitor the increasing CO2 in the atmosphere, and found that the accumulation of the gas was speeding up. While Keeling originally estimated the rate of increase as 0.6 ppm per year, at present this rate is closer to 2.5 ppm per year.
As Arrhenius’s critics correctly pointed out, there are additional factors controlling global temperature. We have already discussed some of these – including the ice albedo feedback articulated by James Croll, and the water vapour feedback set out by Arrhenius himself. In the second half of the 20c, a baffling array of additional, more complex feedbacks were discovered by scientists. Aside from the heavy hitters, such as warming leading to more water vapour leading to more clouds leading to less sunlight reaching the ground leading to cooling (i.e. cloud albedo feedback), there are other, more subtle interactions between C concentrations and global temperatures to consider. To give just one example, scientists have recently found that when levels of CO2 in the atmosphere rise, plants respond by slightly thickening their leaves. While we don’t yet know why they do this, we do know that in so doing they become less efficient at sucking C out of the atmosphere. Higher C levels thus lead to a less efficient removal of C from the atmosphere. So, when atmospheric C levels are elevated for whatever reason, there is an additional positive feedback – in addition to water vapour, ice albedo, and so on – pushing C levels even higher.
The first group, headed up by James Hansen (1941–), showed that by 1980 the world had warmed by 0.2 °C compared to pre-industrial temperatures. This was a small signal, nearly swamped by year-to-year variability. There was also a small cooling trend from 1940 to 1960, which was quite clear in the data…Hansen’s group identified that the N. hemisphere had experienced widespread cooling in the mid-century, bucking the trend of overall warming. Meanwhile, the S. hemisphere had consistently warmed…To organise and collate all this research, the World Meteorological Organization and the UN environmental agencies created the IPCC in 1988.
IPCC reports – and we’ve had six major ones to date – are written by scientists, who, incidentally, are unpaid for this work, and these reports are then subject to line-by-line scrutiny by representatives of national governments. In other words, the IPCC was, and is, an interesting hybrid political and scientific organization, condensing research conducted by scientists into periodic reports curated by politicians.
2022: Earth’s CO2 concentration is 414 ppm, an increase of nearly 50% relative to the pre-industrial concentration of 280 ppm. Accompanying this change in CO2 concentration has been a warming across the globe of, on average, approximately 1 °C.
Global Warming: The increase in global average temperature.
Climate Change: Climactic changes that impact rainfall patterns, the frequency of extreme weather events, average sea levels, the severity of storms, etc.
Global warming is abstract; climate change is experienced.
Summer being one degree Celsius warmer than it was for our great-great-great-great grandparents hardly sounds like a serious issue. It is not experienced.
In ways that science is still struggling to comprehend, the atmosphere appears to be responding to our C emissions. In some places this brings more rain, in others much less. In many places it raises the sea level, flooding coastal areas and forcing people inland. Species of animals are migrating en masse as their previous ranges become uninhabitable, or disappearing altogether. Diseases are finding new localities to spread to. Facing scarcity, humans find themselves forced to migrate. International tensions over fresh water and arable land rise and rise.
The last time C concentrations were as high in the atmosphere as they are now was during the Pliocene, some 3 Ma. During this time, global temperatures were 3 or 4 °C warmer, and sea levels 20m higher than current…Given enough time, the atmosphere – at current CO2 concentrations – will equilibrate at a temperature similar to those in the Pliocene.
Fully half of all energy we use as individuals is ultimately used for heating and cooling, and only 10% of this energy currently comes from renewable sources.
___________________________________________________________________________
Misc Quotes
“Science is a process built on data. In the scientific method, one develops a hypothesis and then tests it against data. If the data supports the hypothesis, it can be considered correct until subsequent data repudiates it.”
___________________________________________________________________________
Terminology
Adiabatic Cooling: The cooling of air as it ascends in the atmosphere.
Atmospheric Science: The study of how layers of the atmosphere interact with one another.
Barometer: Measures the pressure the air exerts.
Brewer-Dobson Circulation: A planet-wide overturning circulation in the stratosphere.
Buys Ballot Law: In the Northern hemisphere, if you turn your back to the wind, the atmospheric pressure is low to your left and high to your right.
Climate: The long-term average (~30yrs) of atmospheric conditions (‘weather’)
Cyclone: A rotation of the fluid of the atmosphere around a point; from the Greek kúklos- ‘circle’.
Depth Sounding: The measurement of a depth of water (from the Old English ‘sund’- swimming).
Earth’s Circumference: ~40,075 km (Polar Circumference: ~40,008 km).
Eötvös Effect: The velocity required for an object to counter the effect of gravity and reach orbit (named after Hungarian physicist Baron Roland von Eötvös (1848–1919)); ~17,400 mph.
Equation of State (Atmosphere): The air pressure at a location (p) is equal to the temperature at that location (T) multiplied by air density (ρ) and the physical constant Rs.
Erratics: Boulders comprised of different rock to their surrounding geology; carried by the action of glaciers.
Etesians: One example of winds that form in a particular place at a particular time of year- other examples in Europe include the Sirocco in the Mediterranean, the Mistral in France, and the Llevantades in Spain; from Greek for ‘periodic’ wind.
Exosphere: The edge of the final, outermost layer of the Earth’s atmosphere. This is nothing more than a scant few molecules still clinging to the Earth’s gravity, barely interacting with one another at all. At around 10,000 km above the surface, the force that sunlight exerts on these rarefied atoms starts to outweigh the force of the Earth’s gravity, and the planet loses its last tenuous grip on the molecules.
Fluid: A general term in physics that encompasses how liquids like water move, how gases like N and O flow, and even how plasma in the heart of the Sun sloshes around. A fluid is a substance made of molecules and atoms, like solid objects, but made of molecules and atoms that only weakly interact with each other.
Geology: The study of the Earth (Greek).
Globalization: The Integration of people, companies, and governments across the world.
Kármán Line: The top of the atmosphere; the edge of space, at ~100km above Earth; partly informed by how high an aircraft could fly in a straight line before travelling into space (named after aeronautics theorist Theodore von Kármán (1881–1963)). According to the Fédération Aéronautique Internationale, anything that takes place under the Kármán line is classed as aeronautics, while anything that takes place above it is astronautics.
Lapse Rate: Change in atmospheric temperature with altitude.
Murmuration: A flock that moves together as a single, colossal entity, flowing between buildings and parting like the Red Sea to evade a diving hawk.
Paleoclimate: The climate of Earth in the distant past.
Planisphere: An articulated map of the sky.
Proto-Globalization: The period in which the Columbian exchange and other colonial activities financed scientists and scientific institutions around the world.
Shu: The Egyptian god of air and wind.
Specific Ideal Gas Constant for Dry Air: ~287 J kg-1 K-1.
Teleconnections: Influencing weather at a great distance, and sometimes with a larger lead time.
Temperature: The average kinetic energy of molecules in a substance. Increasing the temperature of a substance gives each component molecule more kinetic energy; i.e., each molecule in the substance wiggles around more vigorously, and to give themselves enough space they maintain a larger average separation with one another.
Thermometer: Measures the temperatures of the surrounding air.
Trade Winds: Permanent features of the Earth’s tropical regions, where the atmosphere near the surface consistently flows from east to west.
Troposphere
The troposphere extends much higher at the equator than it does at the poles. In low-latitude regions (equator) the troposphere ends at around 17 km, while in high-latitude regions (poles) it tops out at as little as 8 km.
1 km above the north pole the air pressure is significantly lower than the air pressure 1 km above the equator.
Tropopause (‘end of tropos’): The boundary between the lower stratosphere and the upper troposphere; varies in altitude, being higher near the equator and lower near the poles.
Thermopause: The height below which the constituent gases of the atmosphere are well mixed, and above which they are instead stratified by how heavy the molecules are: the heaviest molecules in a layer at the bottom, with H in a layer on the very top. Another definition is the top of the thermosphere, at around 600 km in altitude, above which there are plenty of molecules – H and CO2 mostly – that are still gravitationally attracted to the Earth, but there aren’t enough of them to behave like a gas any more.
Vergeltungswaffe 2 (V2) (‘Retribution Weapon 2’): Designed by German scientist Wernher von Braun as the first large-scale liquid-fueled rocket. It was not of much use as anything other than a psychological weapon- more people were killed in its construction than by its use.
Weather: The variation of atmospheric conditions – rain, sun, cloudy, fog – as experienced on short timescales; what the atmosphere does day to day, week to week.
Wind: Our atmosphere’s way of transporting material around the world; occurs due to changes in pressure over large distances (horizontal pressure gradients).
Pressure Gradient Force: The force that drives flow in the atmosphere.
Geostrophic Wind: Wind that flows along isobars (lines of constant pressure); geo- relating to the earth (Greek) + strophe- twisting/turning (Greek).
___________________________________________________________________________
Chronology
2100: Atmospheric CO2 will likely peak between 500-600 ppm, leading to a global warming of ~2 °C. Expected changes include average sea levels rising by 2m, CAT 5 hurricanes doubling in frequency, several billion people losing ready access to fresh water, tropical diseases becoming far more common further from the equator, an increase in migration and resource-based conflicts, and a mass extinction of plant and animal species occurring on a scale that has not been seen while humans have existed on Earth (Firmament by Clark).
2022: Earth’s CO2 concentration is 414 ppm, an increase of nearly 50% relative to the pre-industrial concentration of 280 ppm. Accompanying this change in CO2 concentration has been a warming across the globe of, on average, approximately 1 °C (Firmament by Clark).
22 Feb- 5 Mar, 2018: A polar vortex known as Anticyclone Hartmut (‘the Best from the East’) strikes the British Isles. For two weeks, frigid air transported all the way from Siberia sat over Europe, causes temperatures to fall to -14 °C in Scotland. This cold air mass was then struck in the west flank by a moisture-laden storm from the Atlantic, producing huge amounts of snow and ice, killing 955, and causing ~£1.2bn in damage (Firmament by Clark).
2002: Japanese researchers set the altitude record for a balloon at 53 km (Firmament by Clark).
1999: Scientists discover that when the N. hemisphere polar vortex breaks apart in sudden stratospheric warming (SSW) events, the jet stream is diverted (Firmament by Clark).
1996: Cyclone Olivia makes landfall in Australia, recording the fastest wind speeds ever recorded on the Earth’s surface at 113m/s (~400 kph) (Firmament by Clark).
1988: The World Meteorological Organization and UN environmental agencies create the Intergovernmental Panel on Climate Change (IPCC) (Firmament by Clark).
28 Aug, 1985: Hurricane Elena (CAT 3) strikes the US Gulf Coast killing nine and causing $1.3B in damage (Firmament by Clark).
1975: Mathematician James Yorke (1941-) coins the phrase chaos theory (Firmament by Clark).
1963: Keeling et al publishes a report titled ‘Implications of Rising Carbon Dioxide Content of the Atmosphere’, detailing that C concentrations were increasing by 0.7 ppm per year. If this were to continue for a few centuries – less time if the rate were to increase – then the C content of the atmosphere would eventually double, leading to a worldwide warming of up to 3.8 °C (Firmament by Clark).
Mar, 1958: Keeling begins taking daily measurement of CO2 at an observatory on the N slopes of Mauna Loa, Hi (Firmament by Clark).
1950: The first digital meteorological forecast is made by meteorologist Jule Charney (1917-1981) and mathematician John von Neumann (1903-1957) (Firmament by Clark).
1947: A V2 rocket launched from White Sands, NM, reaches some 120 km in altitude. This was sufficient to detect not one but two further layers of the atmosphere for the first time (Firmament by Clark).
1942: The first V2 test flight reaches an altitude of 84.5 km (Firmament by Clark).
1934: A significant quantity of ozone in the atmosphere is first detected, indicating that a dense layer of the gas existed in the stratosphere, but not at higher levels. Ozone was known to absorb UV radiation, which would warm up the air in the vicinity of the gas (Firmament by Clark).
1934: A young Wernher von Braun submits his PhD thesis ‘Construction, Theoretical, and Experimental Solution to the Problem of the Liquid Propellant Rocket’ (Firmament by Clark).
16 Mar, 1926: In the snows of Auburn, Massachusetts, Robert Goddard launches the first rocket fueled by gasoline and liquid O. His singularly understated diary entry for the day reads, ‘Tried rocket at 2.30. It rose 41 feet & went 184 feet, in 2.5 secs., after the lower half of the nozzle burned off. Brought materials to lab’ (Firmament by Clark).
1922: Richardson publishes “Weather Prediction by Numerical Process” detailing a systematic method of forecasting the weather using dynamical equations, almost exactly as modern computer models do (Firmament by Clark).
1909-1910: John Poynting (1852-1914) and Frank Very (1852-1927) coin the term “greenhouse effect” in a series of feuding papers (Firmament by Clark).
1904: Norwegian meteorologist Vilhelm Bjerknes (1862–1951) argues that weather forecasting should be possible using mathematical methods. His ideas evolve into the Bergen School of Meteorology (Firmament by Clark).
1896: Swedish geologist Arvid Högbom (1857-1940) first calculates that human activities were adding roughly as much CO2 to the atmosphere as natural processes already were…He estimated that the total C emitted from burning coal would raise the concentration in the atmosphere by only a thousandth part. But if the emissions continued long enough, or if they increased, then perhaps this could be significant (Firmament by Clark).
1890: American meteorologist Cleveland Abbe (1838–1916) proposes that meteorology is simply the application of the laws of physics involving fluids and thermodynamics to the atmosphere (Firmament by Clark).
1873: ~700 people die in London from air pollution (Firmament by Clark).
5 Sep, 1862: Flight of the Mammoth weather balloon; Glaisher and Coxwell set off from Wolverhampton, UK and ascend to over 8,800m (Firmament by Clark).
1858: Ferrel publishes ‘The Influence of the Earth’s Rotation Upon the Relative Motion of Bodies Near its Surface’. This paper, just 4 pages long, is a true milestone in meteorology, crammed with elegant math- it was the first time that someone had combined all the pieces necessary to predict how air behaves on the surface of the Earth. Using data collated by mathematician and fellow meteorologist James Henry Coffin (1806–73), it combined Newton’s second law (F=mA) with rotational mechanics necessary to calculate the deflection a parcel of air experiences as it moves around a rotating sphere. Ferrel had put numbers to the flow of the atmosphere, and in so doing explained Redfield’s ‘Circular Theory’. Air rushes in to occupy an area of low atmospheric pressure, but in doing this it is deflected by the rotation of the Earth- Coriolis deflection (Firmament by Clark).
1856: Professor Joseph Henry (1797-1878) presents a two-page paper written by Eunice Foote (1819-1888) at the Eighth Annual Meeting of the American Association for the Advancement of Science in Albany. This paper was years ahead of its time, detailing lab experiments that found certain gases were in fact opaque to IR radiation, and that absorption of this radiation by these gases significantly increased their temperature. The gas that was found to produce the greatest warming was carbonic acid (CO2) (Firmament by Clark).
1853: Ferrel publishes ‘An Essay on the Winds and Currents of the Ocean’; seen by some as the first in the field of geophysical fluid dynamics (GFD) (Firmament by Clark).
1835: Coriolis publishes ‘On the Equations of Relative Movement of Systems of Bodies’ (in which he does not reference the atmosphere or the rotation of the Earth)- examining the transfer of energy in general rotating systems, using waterwheels as an example (Firmament by Clark).
1831: Redfield proposes his ‘Circular Theory’ of storms describing hurricanes as a ‘progressive whirlwind’ with a calm centre, rotating anticlockwise (Firmament by Clark).
1776: Watt’s steam engine is first commercially installed at an ironworks near Falkirk, Scotland. It could drive steamships, factories, pumps, and railway engines. British industry now had the ability to convert coal into raw economic productivity. With ample coal supplies available within the country, Great Britain led the charge into the Industrial Revolution (Firmament by Clark).
1735: Shortly after being elected a Fellow of the Royal Society, Hadley publishes a paper on the subject, ‘Concerning the Cause of the General Trade Winds’. He improves on Halley’s theory by first, dismissing the idea that warm air rushed in from behind the path of the Sun (if this were the case then the whole planet would experience persistent easterly winds). Instead, Hadley asserted that when air was heated in the tropics and ascended, it was replaced by air not from the E., but from both N. and S. of the equator, where the heating by the Sun was marginally less. This air rushed towards the equator, where it converged. Hadley’s second improvement was that the rotation of the Earth would affect these converging air masses (Firmament by Clark).
1714: Polish-Lithuanian instrument maker Daniel Fahrenheit constructs the first reliable Hg thermometer with his scale, still used today, based on three reference points: the equilibrium temperature of an ice/water/salt mix (0o), the temperature at which ice forms on the surface of water (32o), and the temperature measured when ‘the thermometer is placed in the mouth, or the arm-pit, of a healthy man’ (96o) (Firmament by Clark).
1703: ‘The Great Storm’ strikes Britain killing an estimated 8000 people in shipwrecks, with around a fifth of the Royal Navy being destroyed, along with over a hundred deaths on land in England due to collapsing roofs and chimneys. The wind was so fierce that the lead roofing was blown off Westminster Abbey and one worse-for-wear ship was blown fifteen miles inland (Firmament by Clark).
1701-1744: Life of Swedish astronomer Anders Celsius who invents the temperature scale familiar to most people. He defined his scale as having 100 degrees between the freezing and the boiling point of distilled water; ‘centigrade (100 steps- Latin)’. Originally, Celsius defined the boiling point to be at 0 degrees and the freezing point to be at 100 degrees. The scale was inverted around the time of Celsius’s early death at age 42, allegedly by none other than the famous taxonomist Carl Linnaeus, also from Sweden, for use in his greenhouses (Firmament by Clark).
1686: Halley pens ‘An Historical Account of the Trade Winds, and Monsoons, Observable in the Seas between and Near the Tropicks, with an Attempt to Assign the Phisical Cause of the Said Winds’; arguably the first academic paper ever written in the modern field of climatology (Firmament by Clark).
1637: René Descartes publishes La Meteorologie (Firmament by Clark).
1633: Galileo is found by the inquisition to be ‘vehemently suspect of heresy’ and placed under house arrest (Firmament by Clark).
1629-1695: Life of Dutch mathematician Christiaan Huygens who suggests the use of two fixed points – the freezing and boiling points of water – and delineating degrees of separation between these fixed points to define ‘a universal and determinate standard for heat and cold’ (Firmament by Clark).
1627-1691: Life of English scientist Robert Boyle, who attempts to set a fixed point for a temperature scale. For reasons known only to himself, he set this as the temperature at which aniseed oil (which he surrounded the bulb of his thermometer with) ‘begins to curdle’ (Firmament by Clark).
1611: Jerónimo Beaumont (1533–1613), a member of the Spanish nobility, develops a rudimentary steam engine to drain a silver mine near Seville- the earliest recorded industrial use for a steam engine (Firmament by Clark).
1593: Galileo invents the thermoscope, which compares whether a location had become cooler or warmer over time, or whether it was cooler or warmer than another location (Firmament by Clark).
16c: Formation of the Republic of Letters, an international organization of thinkers that allowed for the rapid transmission of revolutionary ideas (Firmament by Clark).
1473-1543: Life of Nicolaus Copernicus who suggests that the Earth, in fact, orbits the Sun, rather than the other way around (Copernicanism). This is where we get the word ‘revolutionary’; Copernicus’s heliocentrism, with the Earth orbiting the Sun, was so radical that the term ‘revolutionary’ was used synonymously with monumental changes (Firmament by Clark).
15c: Portuguese sailors first recognize the ability of ships to sail from Europe to North America by the trade winds in the Atlantic Ocean (Firmament by Clark).
Late 13c: Venetian glassmakers develop perfectly clear glass that is far more resistant to attack by chemicals or sudden changes in temperature by adding the burned ash of certain plants. This made the development of specialist scientific instruments possible – scientists could now isolate chemicals in their laboratories, or construct elaborate devices such as air pumps (Firmament by Clark).
989-1079: Life of Islamic scholar Ibn Muʿādh al-Jayyānī, who first estimates the height of the atmosphere (Firmament by Clark).
965-1040: Life of Ibn Al-Haytham (Alhazen), best known for his treatise on optics and for emphasizing the importance of experiments to evaluate hypotheses (Firmament by Clark).
340 BCE: Aristotle publishes “Meteorologica” (the study of phenomena in the sky) (Firmament by Clark).
384-322 BCE: Life of Aristotle (Firmament by Clark).
387-271 BCE: Life of Theophrastus, Aristotle’s successor (Firmament by Clark).
390-337 BCE: Life of Eudoxus of Cnidus who surmises that the universe is divided into concentric spheres with the Earth at its center. Around the Earth was a terrestrial sphere bounded by the orbit of the Moon. Above this terrestrial region was the celestial sphere (Firmament by Clark).
625-545 BCE: Life of Thales of Miletus, considered the first natural philosopher as well as being credited with inventing the concept of a mathematical theorem and associated proof. Thales travelled the ancient Med extensively and was said to have visited Egypt to witness the annual flooding of the Nile. While the Egyptians explained the flooding as being the result of the god Hapi’s arrival, controlled by the pharaoh, Thales gave a different, natural explanation. According to his theory, northerly winds prevented the Nile from flooding most of the year by pushing the river water upstream. In flooding season, however, this wind disappeared and so the river would burst out, unopposed, into the floodplain (Firmament by Clark).
3500 BCE: The religion of pre-dynastic Egypt prominently features the sky and incorporates rainmaking rituals (Firmament by Clark).
299-252 Ma: Permian period; global temperatures are ~3 °C colder than now (Firmament by Clark).
360-300 Ma: Origin of coal; an order of plants known as the Lepidodendrales (‘scale tree’- Greek), the earliest tree-like plants with large leaves and thick trunks but relatively shallow roots, dominate Earth. Their wood was made of the polymer lignin which, together with cellulose, gave the plants the ability to stand tall and rigid, with some reaching 50m in height. When Lepidodendrales were falling in Carboniferous forests, there were no bacteria to break down the lignin. Instead, the fallen wood just sat there, untouched, for millennia. Over time, more and more trees would fall on top of one another and compress the earlier trees, first into peat and eventually into coal. The huge quantities of C buried in the ground during this time is from where the Carboniferous Period takes its name – and while coal formation continued for hundreds of millions of years after, it’s estimated that some 90% of the coal we use for fuel today comes from these ‘coal forests’ (Firmament by Clark).
500 Ma: Earth’s atmosphere is recognizably much as it is today- overwhelmingly N with a healthy dollop of O, and a few trace gases such as water vapour, CO2, argon, and several others (Firmament by Clark).
541-485 Ma: Cambrian period; global temperatures are as ~14 °C higher than the present day (Firmament by Clark).
3.5 Ga: Dating of the earliest direct evidence of single-celled organisms on Earth in the form of microscopic fossils containing isotopes of C that can only be produced via biological processes (Firmament by Clark).
___________________________________________________________________________