The Essentials of Biochemistry by REA
Ref: Research and Education Association (2001). The Essentials of Biochemistry. REA.
_____________________________________________________________________________________
Summary
An overview of Biochemistry.
Chemistry: The study of the structure of matter.
Biochemistry: The study of molecules of matter.
Molecules: Composed of atoms bonded covalently.
Biomolecules: Molecules composed of living organisms.
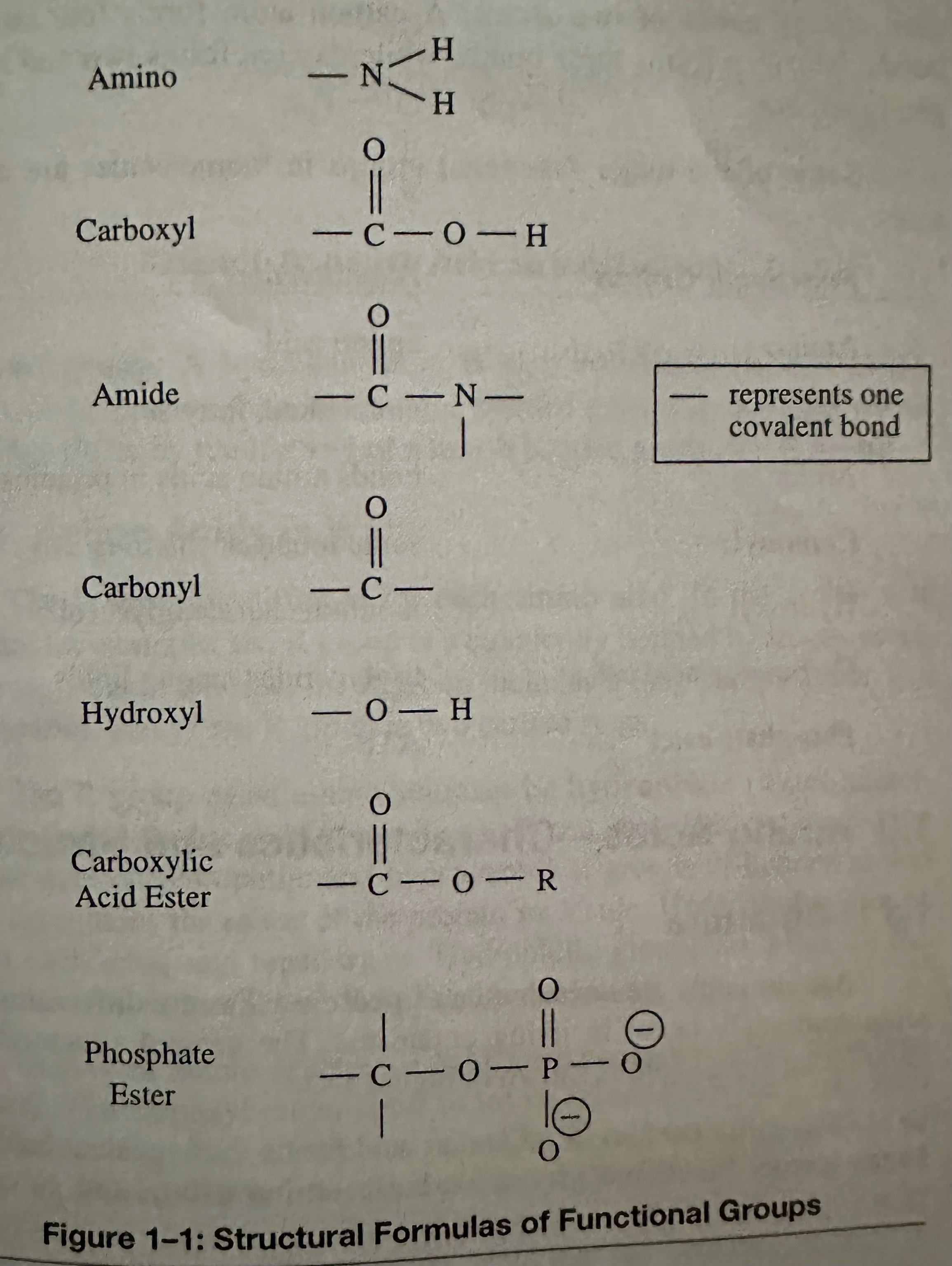
Covalent Bonds: Bonds in which one pair of electrons is shared between the outermost energy shells of two atoms. A C atom forms four covalent bonds. N forms three, O forms two, and H forms one.
Matter: Anything that has mass and volume.
_____________________________________________________________________________________
Cells
There are only two types of cells that compose living organisms; Prokaryotic and Eukaryotic Cells.
Prokaryotic Cells: Small cells with a simple structure. They lack a well-defined nucleus and membrane bound organelles, or cell parts.
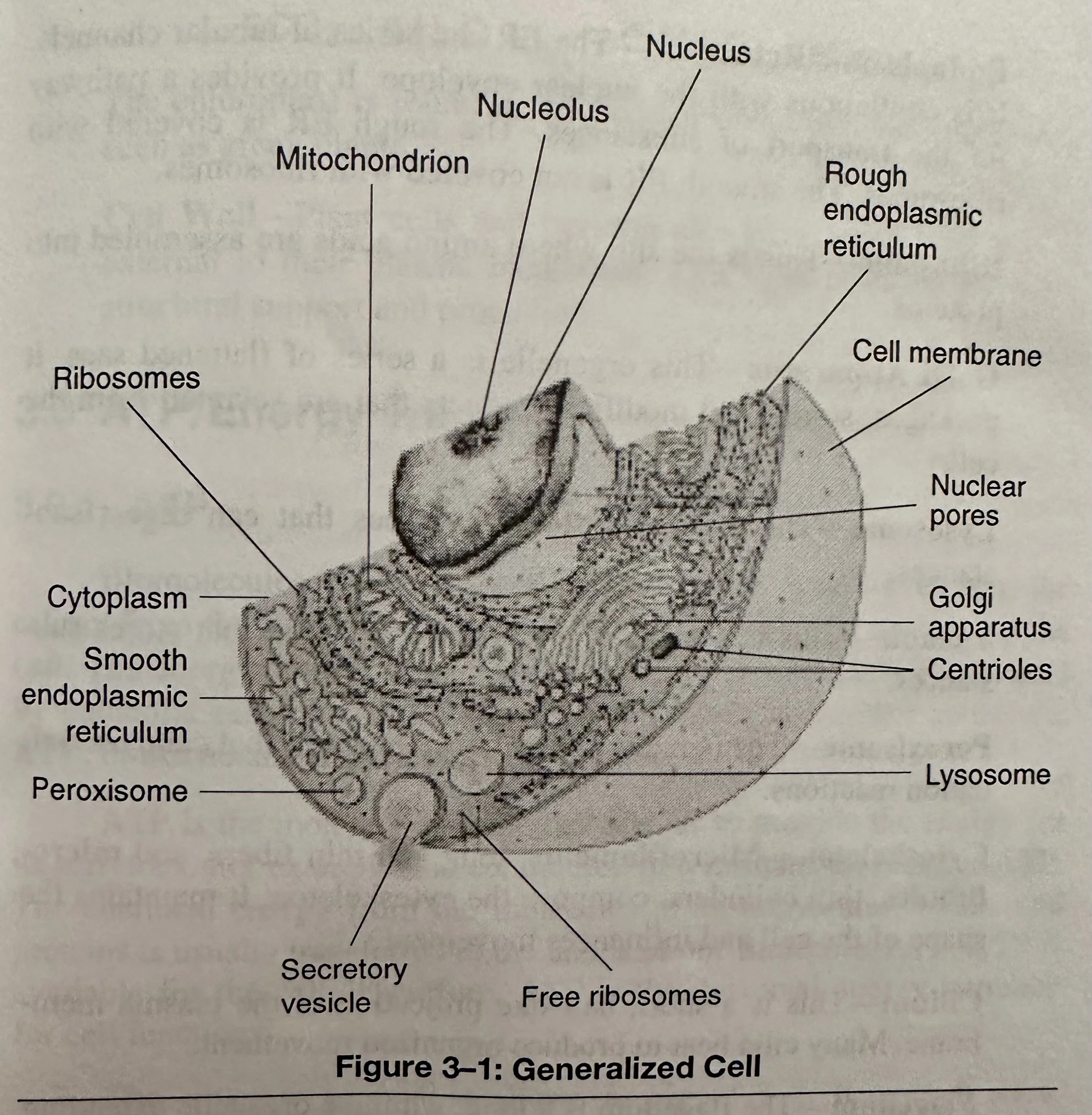
Eukaryotic Cells: Cells of plants and animals that are larger and more complex. They have a membrane bound nucleus and numerous organelles. The organelles are located in the cytoplasm.
Parts of a Cell
Nucleus: Defined by a double membrane, the nuclear envelope. It contains rod-like structures, the chromosomes.
Nucleolus: A spherical body inside the nucleus consisting of RNA and protein. The Nucleoli are made by the chromosomes and compose the ribosomes that participate in protein synthesis.
Ribosome: The site where amino acids are assembled into proteins.
Chromosomes: Carry genes, the units of heredity. The DNA of genes encodes genetic information.
Centriole: A cylinder shaped organelle near the nuclear envelope. Usually there are two at right angles to each other. The centrioles coordinate the events of cell division.
Cytoplasm: The material between the nucleus and plasma membrane of a cell.
Endoplasmic Reticulum: A series of tubular channels that provides a pathway for the transport of substances.
Golgi Apparatus: An organelle that is a series of flattened sacs. It packages, stores, and modifies products that are secreted from the cell.
Lysosome: An organelle that stores enzymes that can digest substances.
Vacuole: A membrane enclosed structure that stores substances.
Peroxisome: Contains enzymes that catalyze oxidation reactions.
Cytoskeleton- Microfilaments: Long and thin fibers and microtubules; thin cylinders composed of cytoskeleton. It maintains the shape of the cell and influences movement.
Cilium: A short, hair-like projection of the plasma membrane. Many cilia beat to produce organized movement.
Flagellum: A long, whiplike, organelle extending from the plasma membrane. It produces movement for the cell.
Mitochondrion: The powerhouse of the cell. It is usually long and oval shaped with a double membrane. The inner membrane has folds, or cristae, that project into the matrix. This divides the matrix into compartments. The matrix and inner membrane contain enzymes and transports molecules for releasing energy from other molecules, such as glucose.
Chloroplast: The sight of photosynthesis; contains the green pigment chlorophyll, which can trap sunlight.
Cell Wall: The rigid covering that provides structural support and protection.
_____________________________________________________________________________________
Carbohydrates
Carbohydrates: A biomolecule consisting of C, H, O, usually with a H: O ratio of 2:1 (as in water) and thus with the empirical formula Cm(H2O)n.
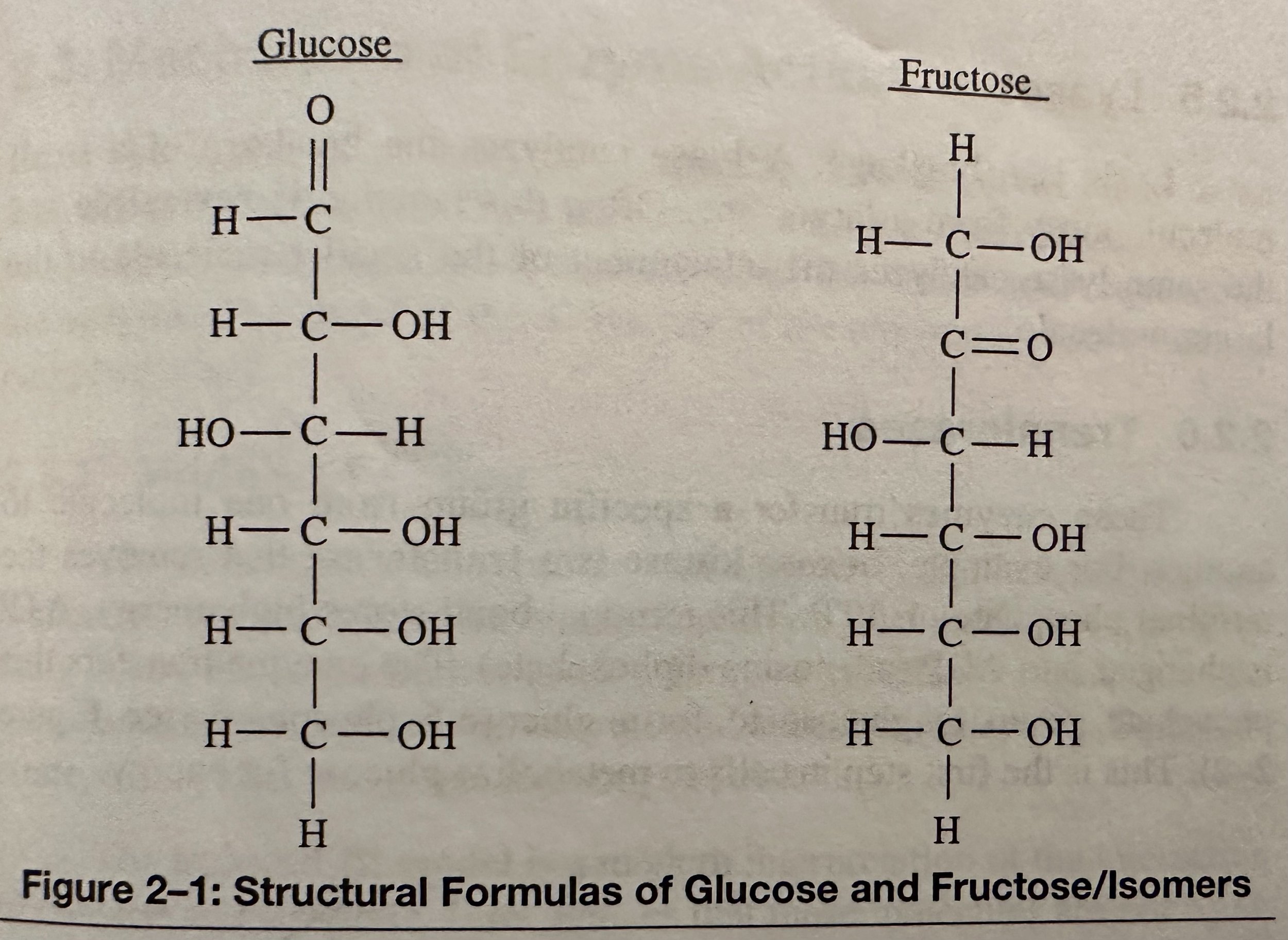
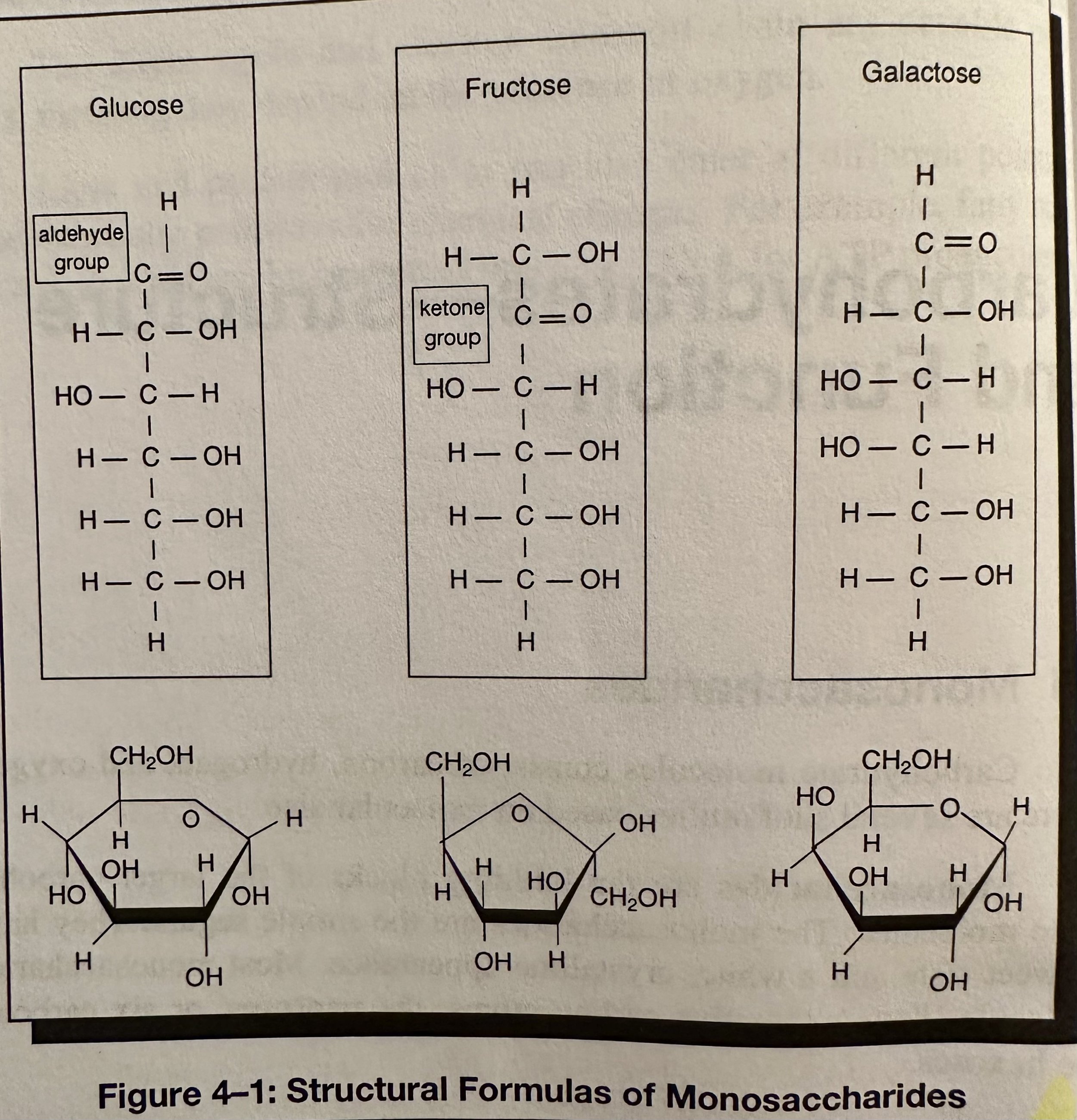
Monosaccharide: A simple carb with molecules of glucose, fructose, and galactose (simple sugars with the same formula- C6H12O6, but different structural formulas); the building blocks of the larger carb molecules. They have a sweet taste and a white, crystalline appearance. Most monosaccharide molecules have either 5 C atoms (pentoses) or 6 C atoms (hexoses).
The major biological role of monosaccharides is to provide an immediate source of energy for cells. In the human body, glucose is dissolved in the plasma, the liquid part of the blood. When accepted by cells, it is chemically changed to make ATP.
Disaccharide: A simple carb; a combination of two monosaccharides by a dehydration synthesis. Important disaccharides are maltose (2x glucose), sucrose (glucose + fructose), and lactose (glucose + galactose). Disaccharides provide a source of energy for cells. Maltose is malt sugar, Sucrose is table sugar (the major plant sugar), and lactose is a milk sugar.
Dehydration Synthesis (‘Condensation Reaction’): C6H12O6 + C6H12O6 = C12H22O11 + H2O.
Hydrolysis: The breakdown of a disaccharide into monosaccharides by the insertion of a water molecule (the reverse of dehydration synthesis). By Hydrolysis, sucrose is digested into glucose and fructose. Lactose is digested into glucose and galactose. Each hydrolysis reaction requires a separate enzyme.
Polysaccharide: Complex carbs; a large molecule consisting of multiple monosaccharides combined by a dehydration synthesis.
Starch: A complex carb; the major polysaccharide that stores energy in plants; consists of glucose molecules bonded in a chain.
Glycogen: The main polysaccharide storing energy in many animals and the human body. Glycogen consists of many glucose molecules bonded together and is stored in the liver and skeletal muscles. The human body can store only about 400-500g of glycogen.
Glycogenolysis: The breakdown of glycogen into glucose at its storage sites in the body, where it serves as an immediate source of energy for cells. It is promoted by the hormone glucagon, which is produced by the pancreas.
Glycogenesis: The conversion of glucose into glycogen when too much glucose is present in the blood. This process is promoted by Insulin, a hormone produced by the pancreas.
Cellulose: A complex carb and the major structural polysaccharide of plants; consists of bonded glucose molecules. Humans lack the enzyme necessary for hydrolyzing the bonds connecting glucose in cellulose. Therefore, cellulose cannot be digested by humans if present in their diet.
Derivatives: The combination of polysaccharides with other molecules. Derivatives include glycolipids and glycoproteins.
Chitin: A polysaccharide derivative found in the skeletons of crustaceans and insects.
Chemical Digestion of Carbs: In humans, polysaccharides and disaccharides in the diet are chemically digested to . monosaccharides so they can be metabolized for ATP formation.
Starch Digestion: The hydrolysis of starch begins in the oral cavity, there enzyme salivary amylase converts this polysaccharide into maltose. Starch continues on to the stomach the small intestine where Amylase secreted from the pancreas finishes changing starch into maltose. Maltose and other disaccharides are hydrolyzed into their monosaccharides.
Each chemical change requires a specific enzyme. Maltase is the enzyme that converts the substrate maltose. Sucrase is the catalyst for Sucrose. Lactase works on lactose.
Monosaccharides produced by chemical digestion are absorbed from the small intestine into the bloodstream. They are transported to the liver for additional metabolism. The blood continually delivers glucose to the cells throughout the body. In some cells, the glucose is converted to glycogen and lipids for energy storage. In many cells, however, glucose is metabolized for ATP formation.
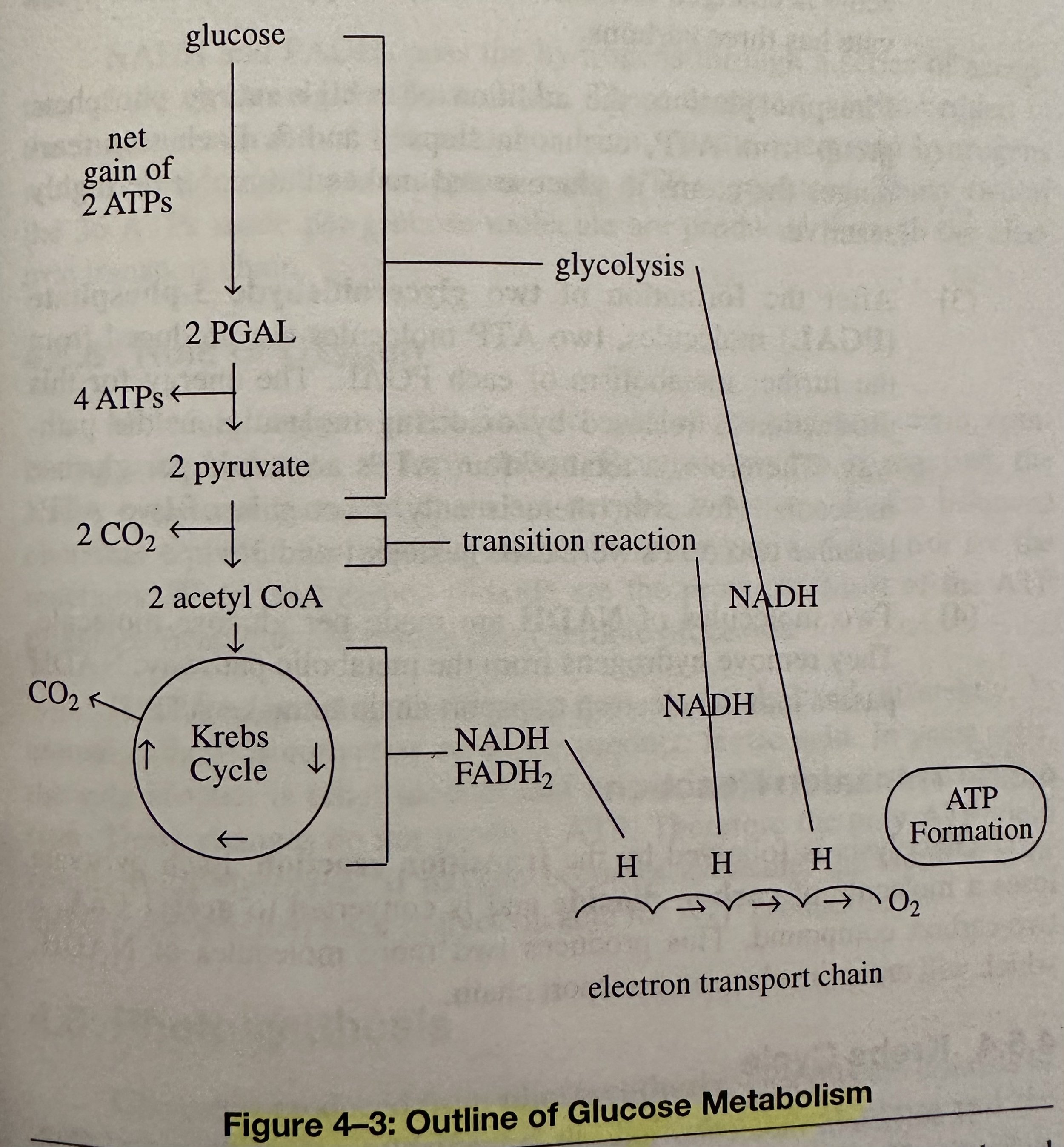
Glycolysis: The first state of metabolism for glucose. This stage is anaerobic, meaning that it does not require the presence of molecular O.
Pyruvate Metabolization: If O is not available during pyruvate metabolization, it forms lactic acid in animal cells and ethyl alcohol, via fermentation, yeast cells.
Gluconeogenesis: The metabolism of a substance other than glucose for energy.
_____________________________________________________________________________________
Proteins
Proteins: Large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalyzing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells, and organisms, and transporting molecules from one location to another. Proteins differ in their sequence of amino acids, which is dictated by the nucleotide sequence of the genes. 1g of Protein ~ 4 Kcal. Proteins have four levels of structure.
Primary Structure: The sequence of amino acids covalently bonded together. With 20 amino acids in cells, the possibilities for different sequences are endless.
Secondary Structure: The result of the amino acid sequence of the polypeptide.
Tertiary Structure: The 3D folding of the alpha-helix or the pleated sheet.
Quaternary Structure: The spatial relationship between the different polypeptides in the protein.
Major Bio functions of proteins:
Structure: Proteins compose the makeup of hair, bones, and muscles (I.e., Keratin, Collagen).
Regulation: Some hormones are proteins. Hormones act as chemical messengers transported in the blood; they control body processes.
Transport: Hemoglobin; transports O in the blood.
Contraction: Actin and Myosin are contractable proteins in muscles.
Catalysts: All enzymes, which are organic catalysts, are mainly protein.
The intake of proteins contributed to the N balance of the body.
Protein Digestion: The chemical digestion of dietary proteins begins in the stomach, which is highly acidic (pH of 1-2) from the secretion of HCL, which denatures proteins in the diet. From the stomach, the partially digested proteins enter the small intestine (pH of 8-9) where Hydrolysis of proteins is completed. Hydrolysis of peptide bonds in dietary proteins produce amino acids, which are absorbed into the bloodstream from the small intestine.
Proteases: Enzymes that catalyze protein digestion.
_____________________________________________________________________________________
Lipids
Lipids: A broad group of naturally occurring molecules that contain C, H, and O and includes fats, waxes, sterols, fat-soluble vitamins (Vitamins A, D, E, K), monoglycerides, diglycerides, phospholipids, and others. The functions of lipids include storing energy, signaling, and acting as structural components of cell membranes. Triglycerides store energy efficiently; 1g stores ~9 Kcal.
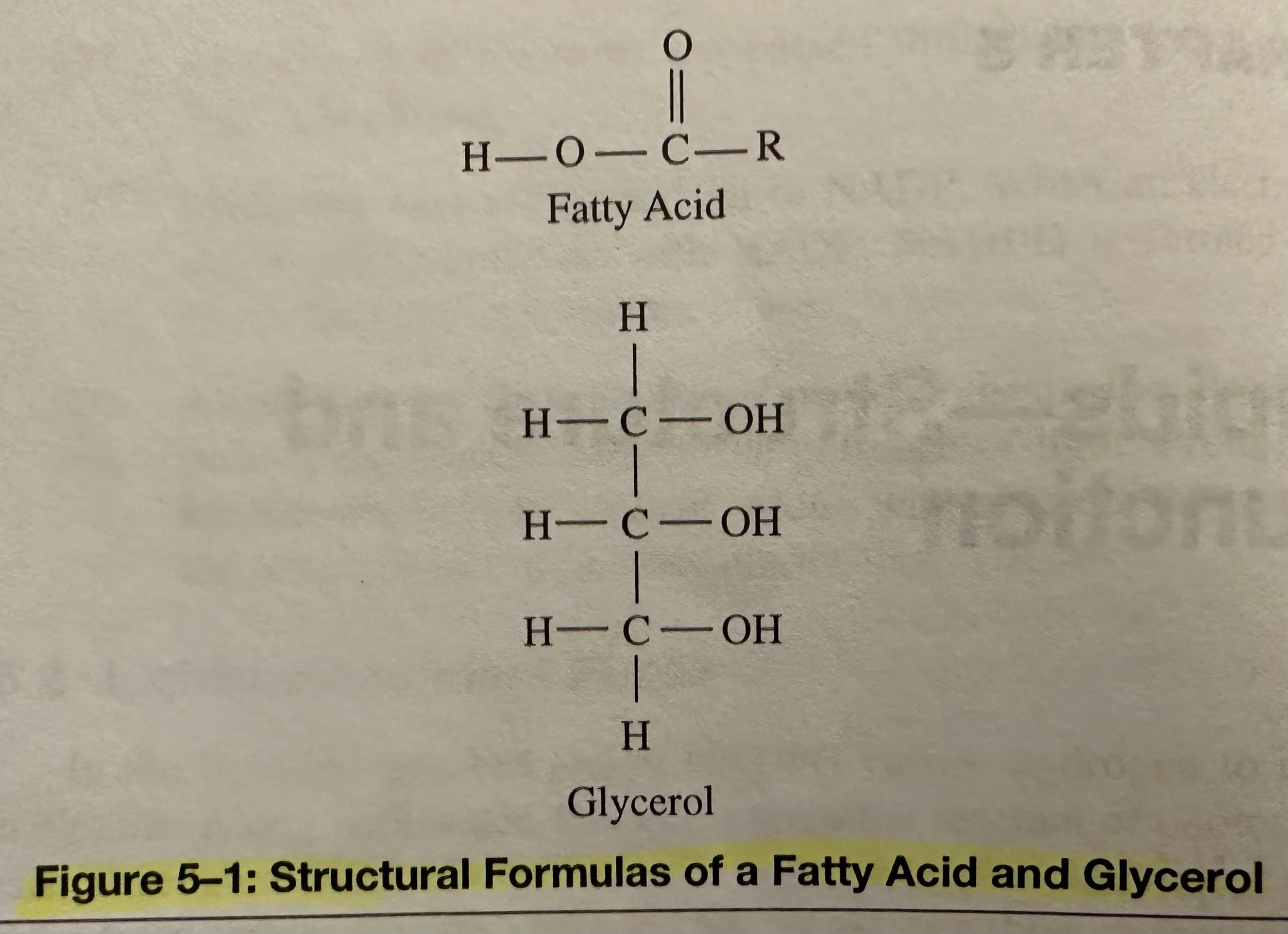
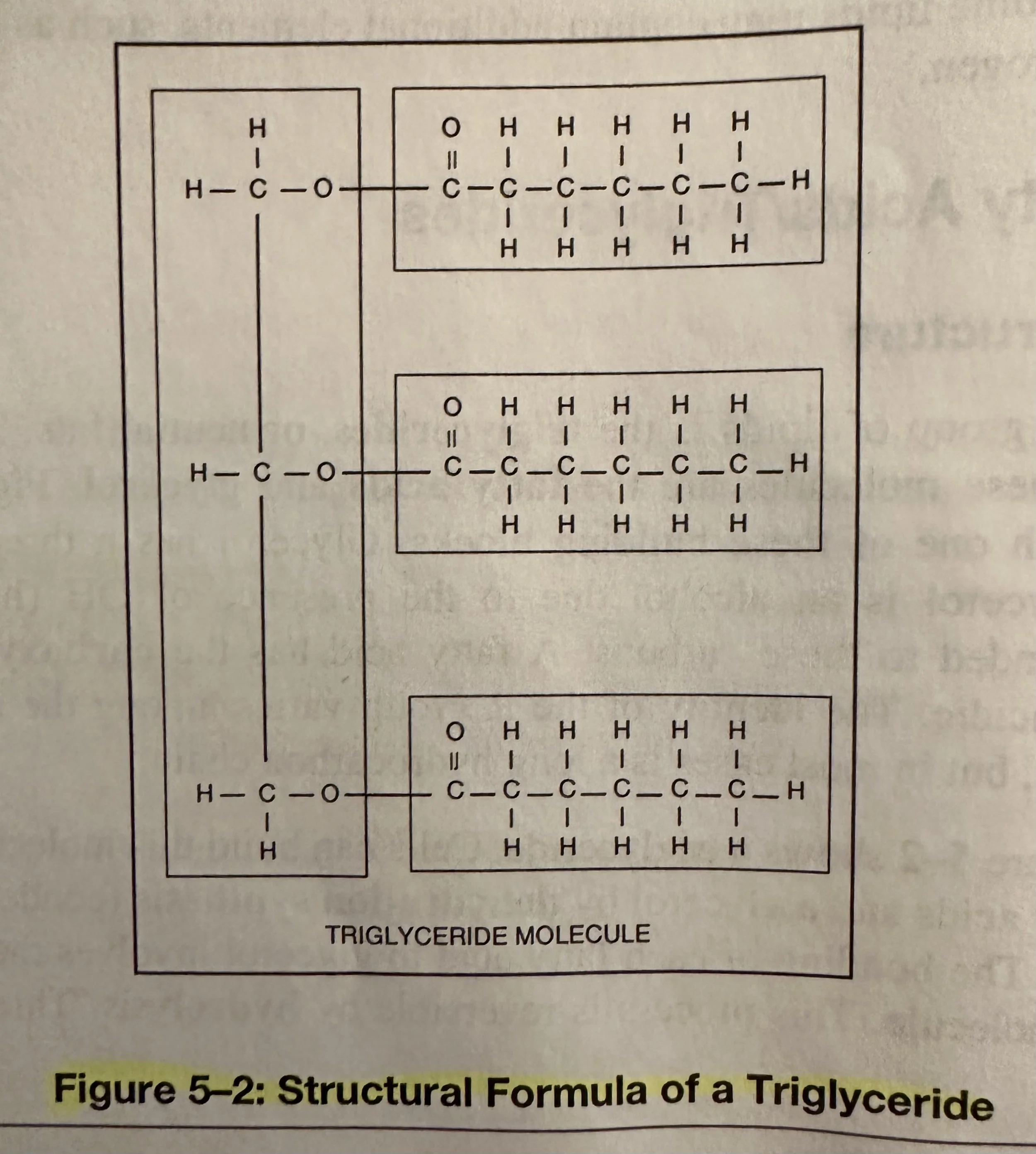
Triglycerides: A type of lipid known as a neutral fat. The subunits of triglyceride molecules are the fatty acids and glycerol. Glycerol and Fatty Acids combine via dehydration (condensation) synthesis to build Triglycerides.
Glycerol: Contains a 3-C chain; an alcohol due to the presence of OH (hydroxyl) groups bonded to the C’s.
Fatty Acid: Contains a carboxyl group, which is acidic. There are many different kinds of fatty acids. They differ by length of the C chain of the R group. The chains can range from 4-24 C’s, but usually with an even number. Fatty acids can be saturated or unsaturated.
Oleic Acid: A fatty acid with 18 C’s.
Palmitic Acid: A fatty acid with 16 C’s.
Saturated Fatty Acid: Contains the max number of H covalently bonded to the C chain of the R group.
Unsaturated Fatty Acid: Contains at least one double bond between C’s in the R group. This decreases the number of H atoms that can bond to the C chain. Therefore, it is not saturated with H. Linoleic Acid and linolenic acid are unsaturated. They are also essential fatty acids for humans, as the human body cannot produce them by metabolism.
Oils: Triglycerides formed from unsaturated fatty acids that are liquid (or oils) at room temperature. They are commonly found in plants.
Fats (Animal): The triglycerides formed from saturated fatty acids are solids at room temperature.
Phospholipids: A derivative of triglycerides, or neutral fat. Phospholipids compose the membranes of cells. For example, the plasma membrane is partly phospholipid. The polar ends of the phospholipid molecules are attracted toward water. The remaining part of these molecules are nonpolar and are oriented away from water.
Lipid Digestion: Lipid digestion in humans begins in the small intestine. Bile secretion, produced by the liver and stored in the gallbladder, starts the process of emulsification. Next, the small lipid droplets undergo enzyme action. Lipase is the enzyme that catalyzes the hydrolysis of lipids into their subunits. The subunits are absorbed into the bloodstream from the small intestine and are transported to the liver for further metabolism.
Emulsification: The breakdown of large lipid masses into smaller droplets by bile in the small intestine.
Lipid Metabolism: Glycerol, supplied from the digestion of lipids or the breakdown of stored triglyceride molecules, is converted into glyceraldehyde-3-phosphate (PGAL). PGAL enters glycolysis for ATP production.
Fatty acids can be converted into acetyl CoA by the breakdown of lipids. Additionally, the human body can produce some fatty acids, the nonessential fatty acids, through lipogenesis. Acetyl CoA passes into the Krebs cycle and the electron transport chain for ATP production.
Ketosis: The conversion of acetyl COA into ketone bodies during fasting for future energy use.
_____________________________________________________________________________________
Amino Acids
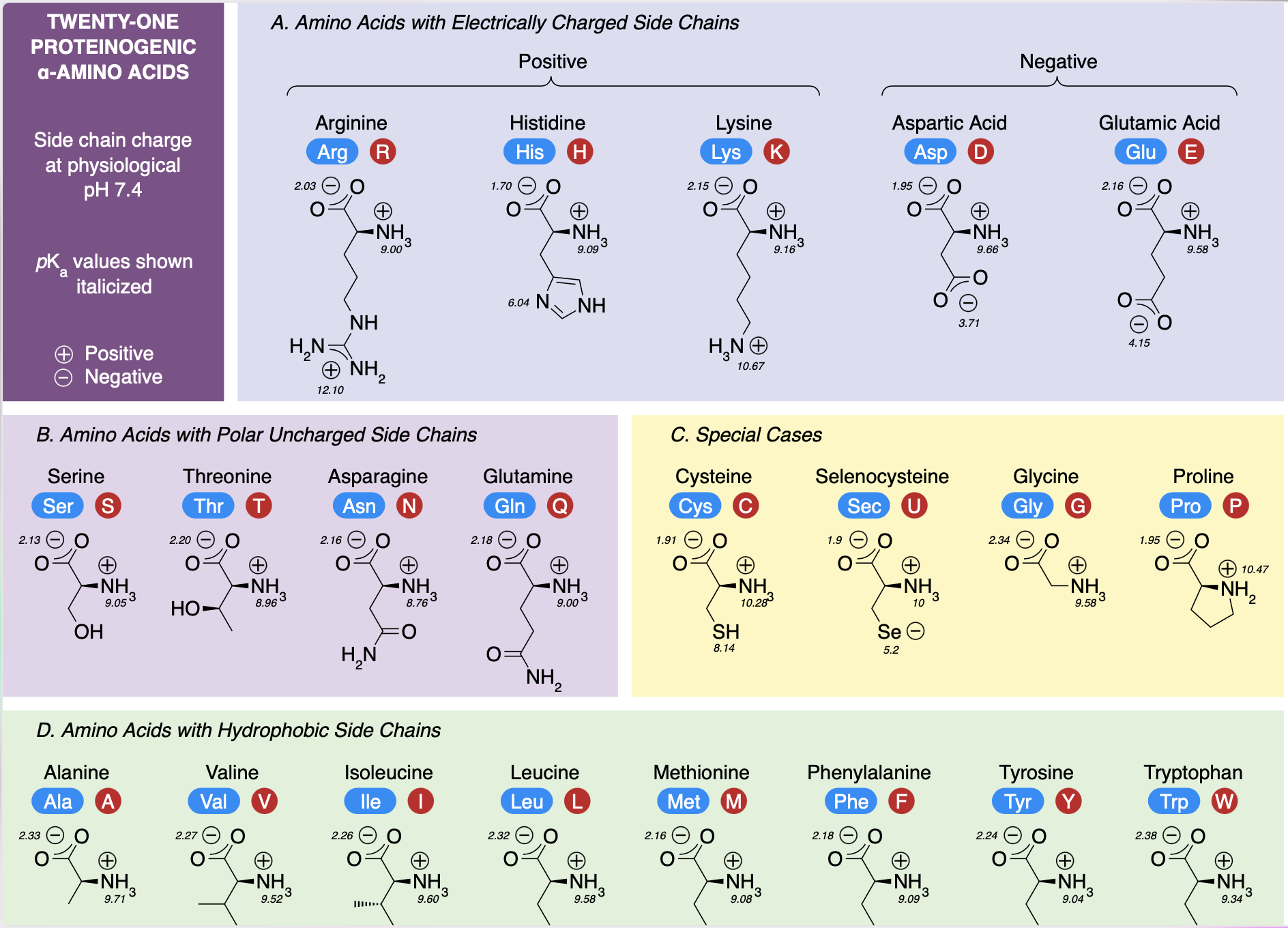
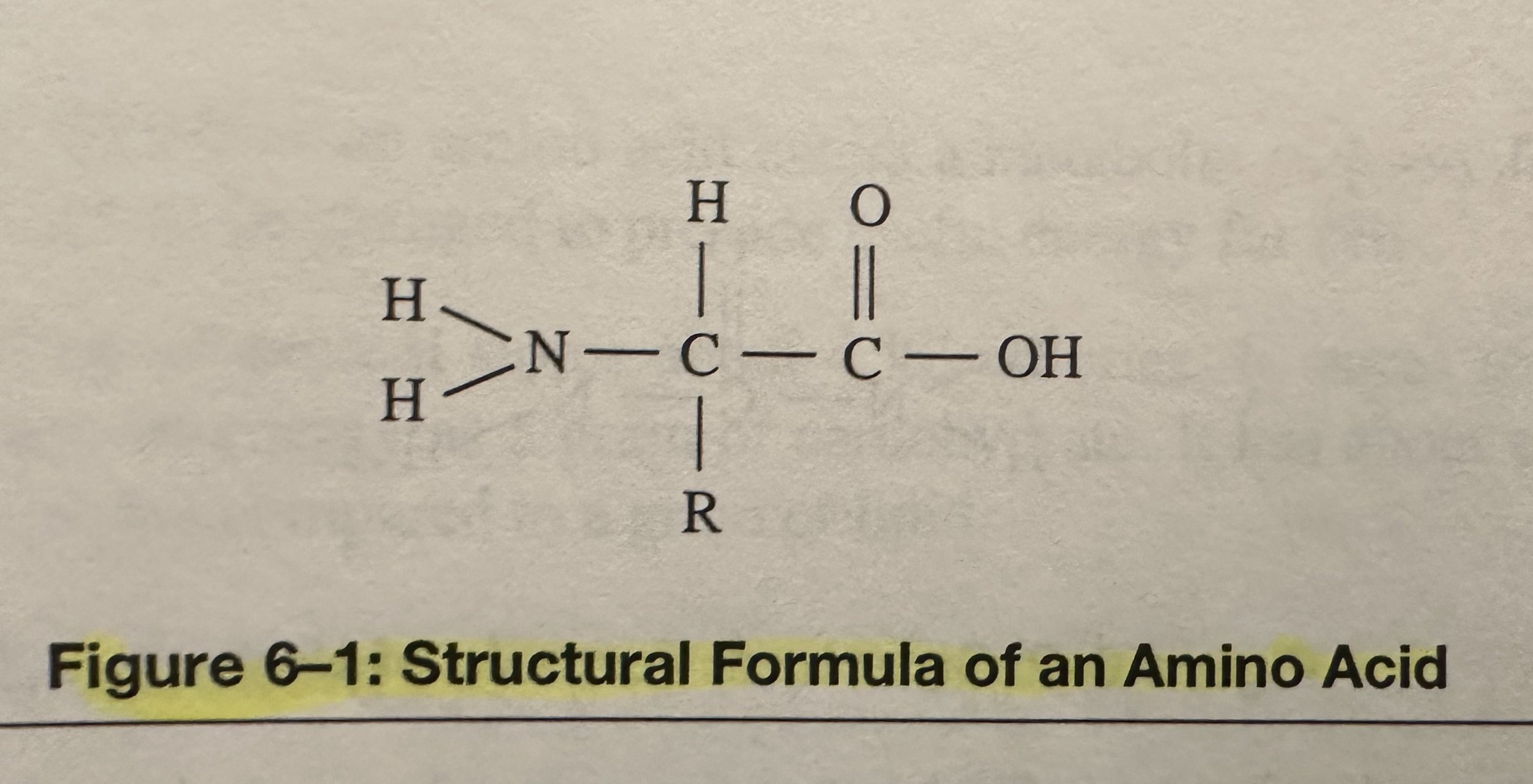
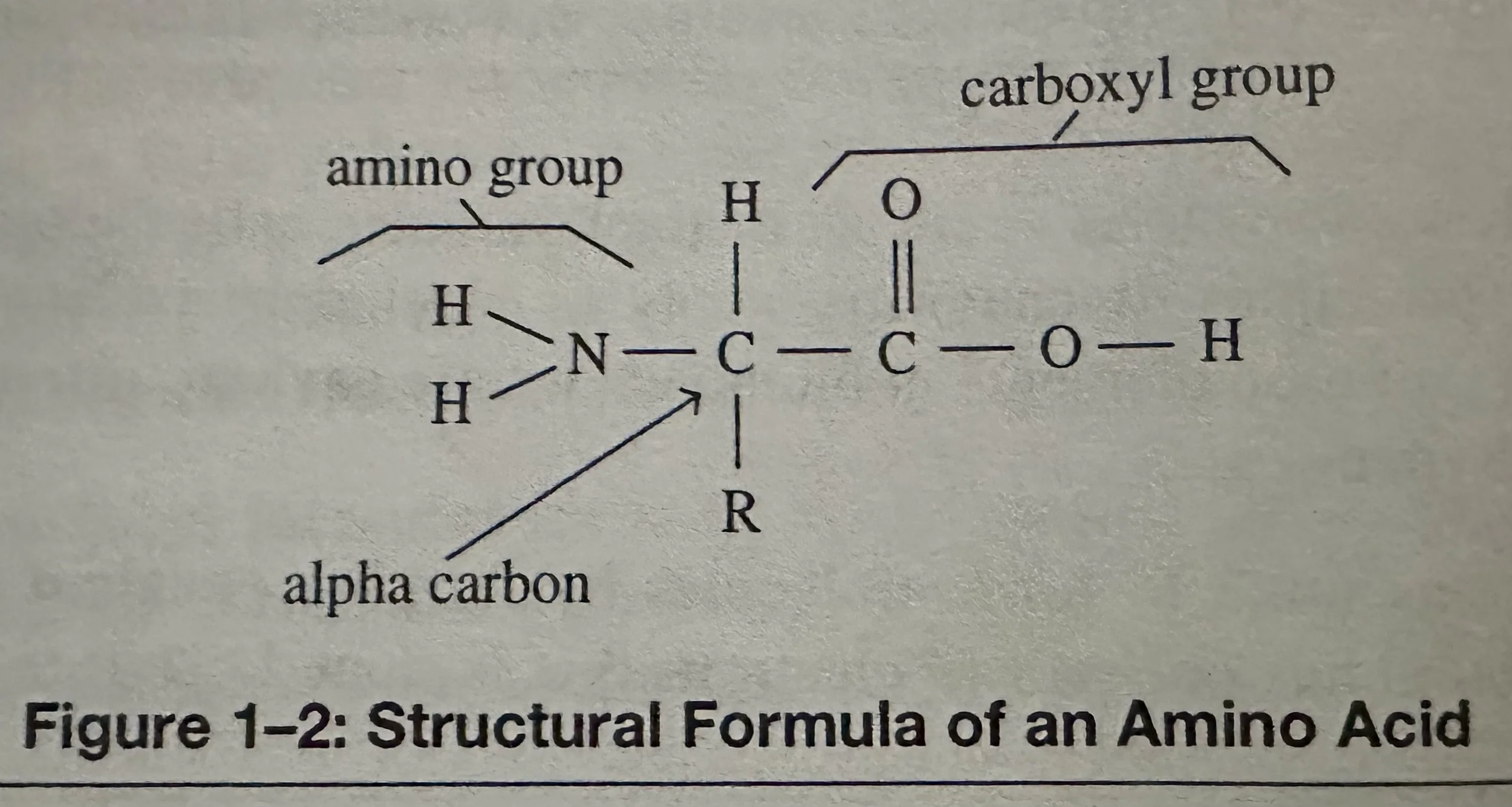
Amino Acids: Subunits of proteins; organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in the nature, the most important are the alpha-amino acids, from which proteins are composed. Only 22 alpha amino acids appear in the human genetic code, of which 20 are used for human metabolism; 11 of these are nonessential and 9 of these are essential.
Nonessential Amino Acids: Amino Acids that can be synthesized by metabolic pathways and are not required in the diet; nonessential amino acids include alanine, arginine, asparagine, aspartic acid, cysteine, glutamic acid, glutamine, glycine, proline, serine, and tyrosine.
Cells have metabolic pathways to make the nonessential amino acids. One of the precursors for this synthesis is pyruvate, which is formed from glucose by glycolysis.
Essential Amino Acids: Amino acids that are produced by plants and microorganisms that cannot by synthesized by the human body. The essential amino acids for humans are required in the diet. They are histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine.
The human body has a metabolic pool of amino acids. Some enter the body from the diet while others are produced from proteins broken down in cells.
Alpha C (of an amino acid): Forms four covalent bonds. It bonds to two functional groups- a basic amino group and an acidic carboxyl group. A H atom is also covalently bonded to this C. Amino acids share these three bonded groups in common, however they differ by the identity of a fourth bonded group, the R group.
R Group (of an amino acid) can be hydrophilic (water attractable) or hydrophobic (water repellant).
An amino acid can have a positive and a negative end. This dipolar ion is called a zwitterion.
Amino Acid Catabolism
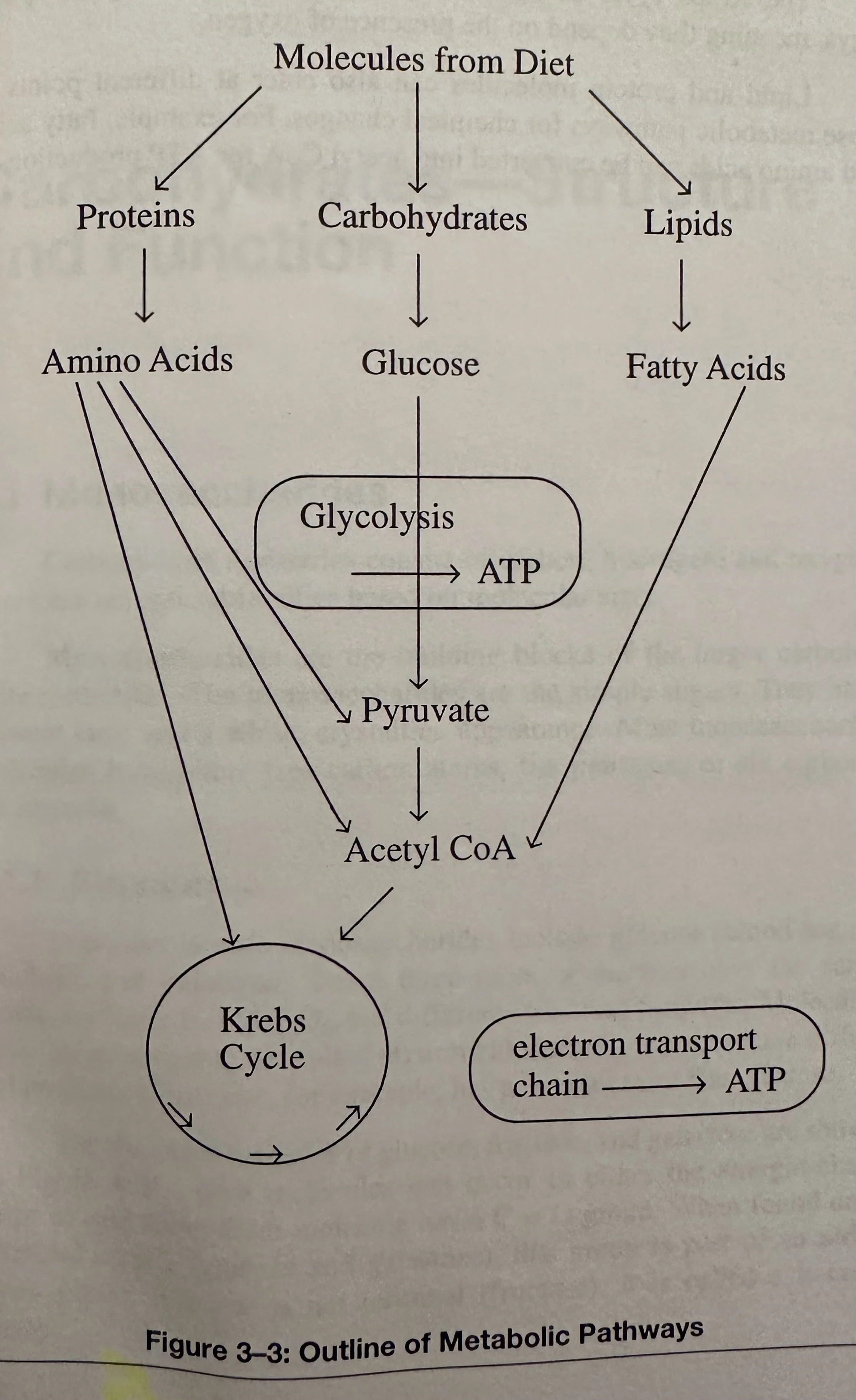
Deamination: The initial step in the catabolism of amino acids. This is the removal of the amino group -NH2 from the Amino Acid molecule. Amino acids pass through the same metabolic pathways that use glucose to produce ATP. After deamination, the remaining part of some amino acids is converted into pyruvic acid. Others are either converted to acetyl CoA or changed into compounds of the Krebs cycle.
Transamination: A chemical reaction that transfers an amino group to a ketoacid to form new amino acids. This pathway is responsible for the deamination of most amino acids and is one of the major degradation pathways which convert essential amino acids to non-essential amino acids.
_____________________________________________________________________________________
Energy
Energy: The ability to perform work. Energy is neither created nor destroyed.
Biomolecules: Molecules that store energy, including carbs. Living organisms metabolize biomolecules and use this energy to carry out their daily activities.
Metabolism: Converts the chemical energy in a glucose molecule to kinetic energy during contraction of a muscle. During this conversion, some of the chemical energy is also converted into heat energy. There are two major patterns of metabolism for biomolecules:
Catabolism: The chemical breakdown of larger molecules into smaller molecules, when the chemical bonds of the larger molecules are broken, energy is released for muscle contraction. Catabolic reactions are exergonic; they release energy.
The energy released from catabolism drives the anabolic changes in cells. For example, the catabolism of glucose can provide the energy source to build proteins.
The energy from biomolecules, such as glucose, must be released by catabolic reactions and trapped in the bonds of another molecule- ATP.
Anabolism: The assembly of smaller molecules into larger molecules. The chemical bonds in these larger molecules store energy. Anabolic reactions are endergonic; they store energy.
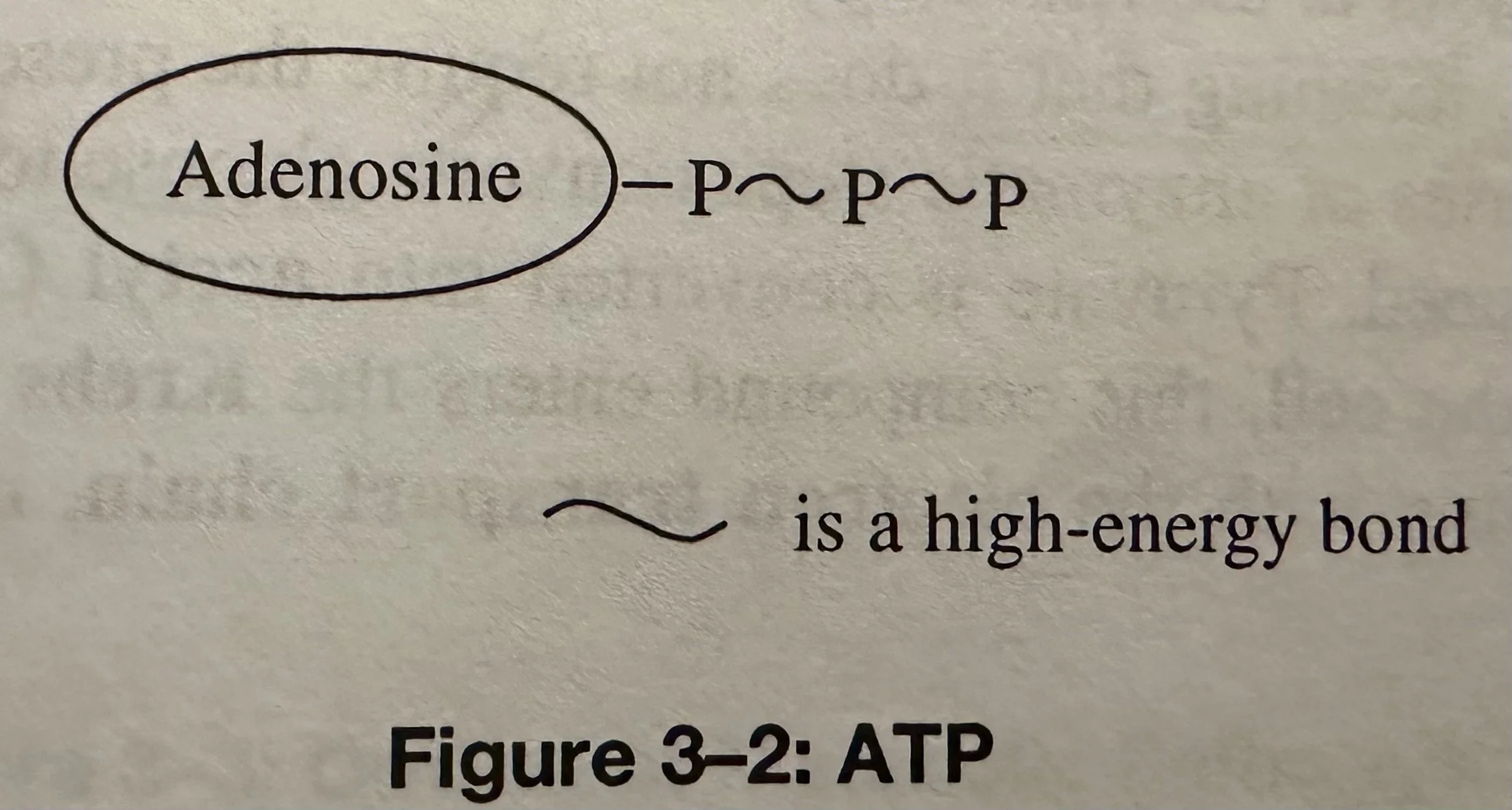
Adenosine Triphosphate (ATP): The Molecule that the cell spends to provide the energy for its activities. ATP consists of a N containing compound (Adenosine), plus 3x Phosphate groups that are bonded in sequence. If either of these bonds is broken, a large amount of energy is released.
Often, only the terminally bonded phosphate group is broken in ATP to release energy in the cell.
Hydrolysis: ATP + H2O = ADP + P + energy.
During prolonged fasting, the body converts all of its stored glycogen into glucose. To continue to produce ATP, the body will usually call on fatty acids and glycerol next. If these molecules become depleted, amino acids are used last. Therefore, there is an order of preference in human catabolism for ATP production: carbs to lipids (glycerol and fatty acids) to proteins (amino acids).
_____________________________________________________________________________________
Enzymes
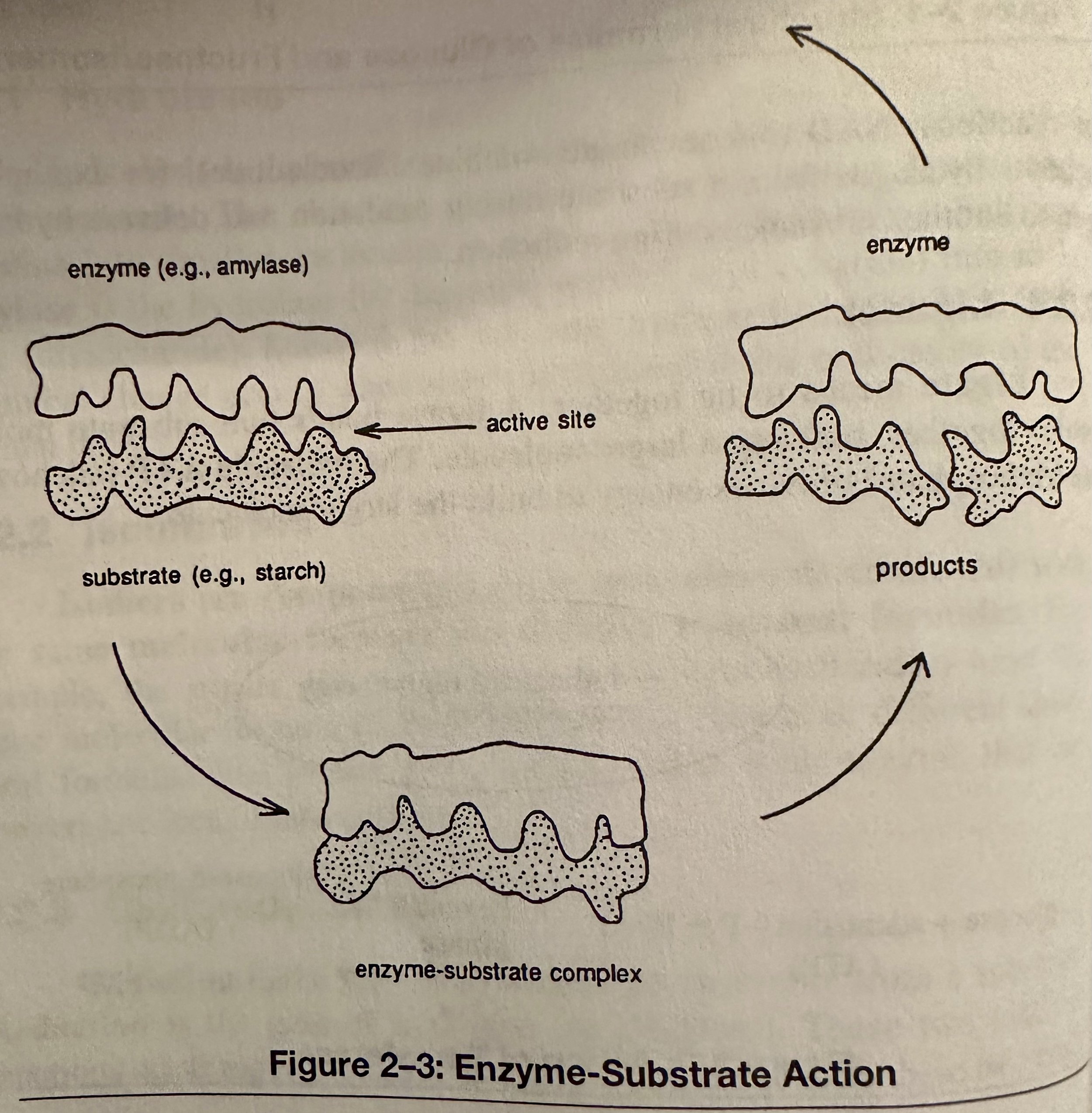
Enzyme: Mainly a protein; must have a specific shape to fit into another molecule and make it reactive. By doing this, an enzyme acts as a catalyst, speeding up the rate of a chemical reaction. The structure of an enzyme determines how it functions. Enzymes control the steps of metabolism.
Catalyst: Increases the rate of a chemical reaction while remaining unchanged in the process.
Cofactor: The nonprotein part of the enzyme; often an organic molecule called the coenzyme, which can be a vitamin.
Substrate: The reactant that an enzyme changes into a product in a chemical reaction.
Enzymes are classified by the kind of reactions they catalyze. There are six major classes with most enzyme names ending in -ase.
Hydrolases: The breaking of a chemical bond by the insertion of a water molecule.
Isomerases: Compounds with the same molecule formula but different structural formulas. An isomerase is an enzyme that can convert one isomer into another.
Oxidoreductases:
Oxidation: The loss of H (or e-) from a substrate.
Reduction: The gain of H (or e-) from a substrate.
Ligases (‘tie together’): Bonds two substrate molecule together, building a larger molecule.
Lyases (‘break’): Catalyzes the breakage of a small molecule away from a larger one.
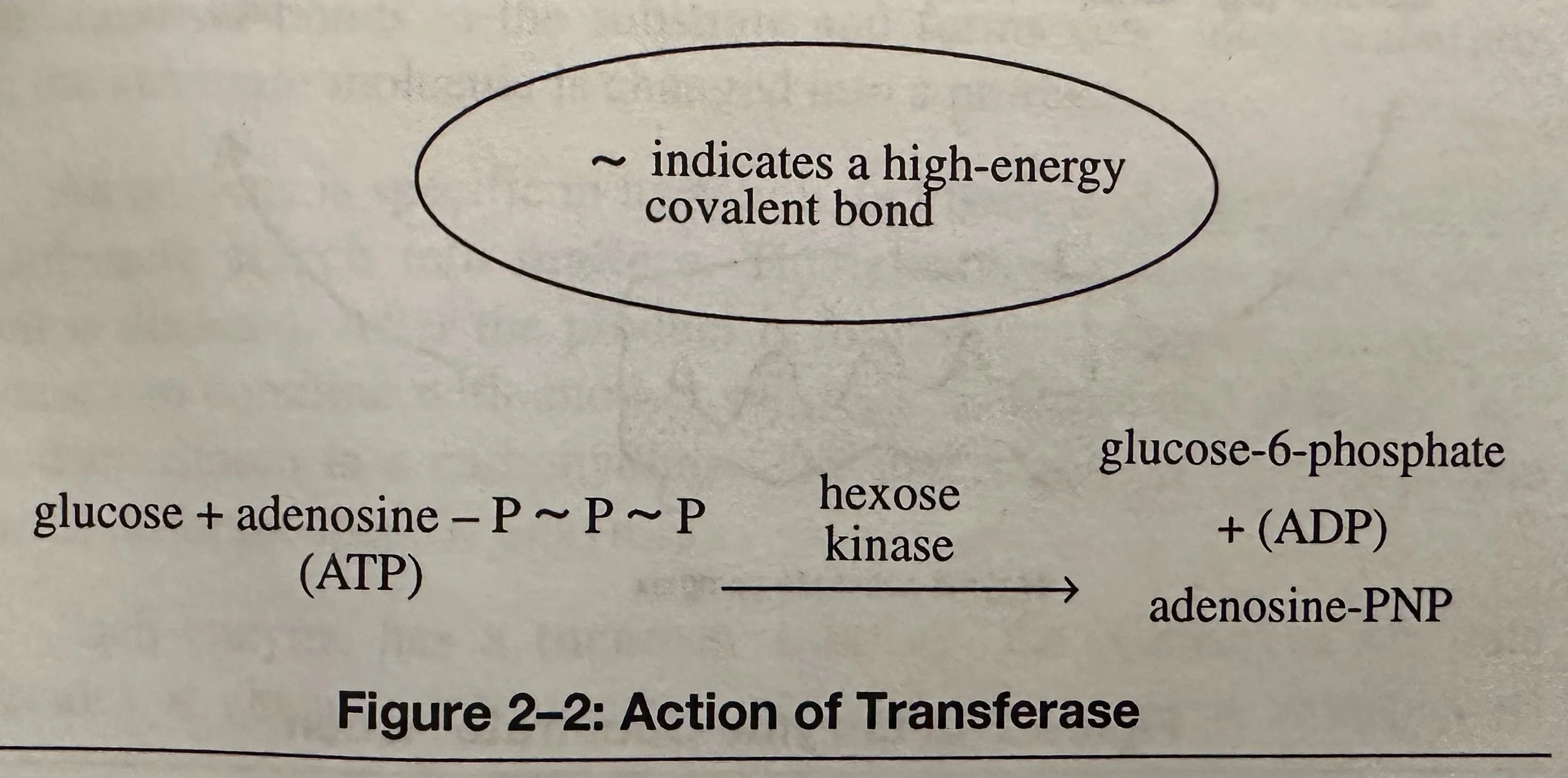
Transferases: Transfers a specific group from one molecule to another.
The action of an enzyme is specific. Enzyme specificity is related to the complementary shapes of the enzymes and substrate molecules.
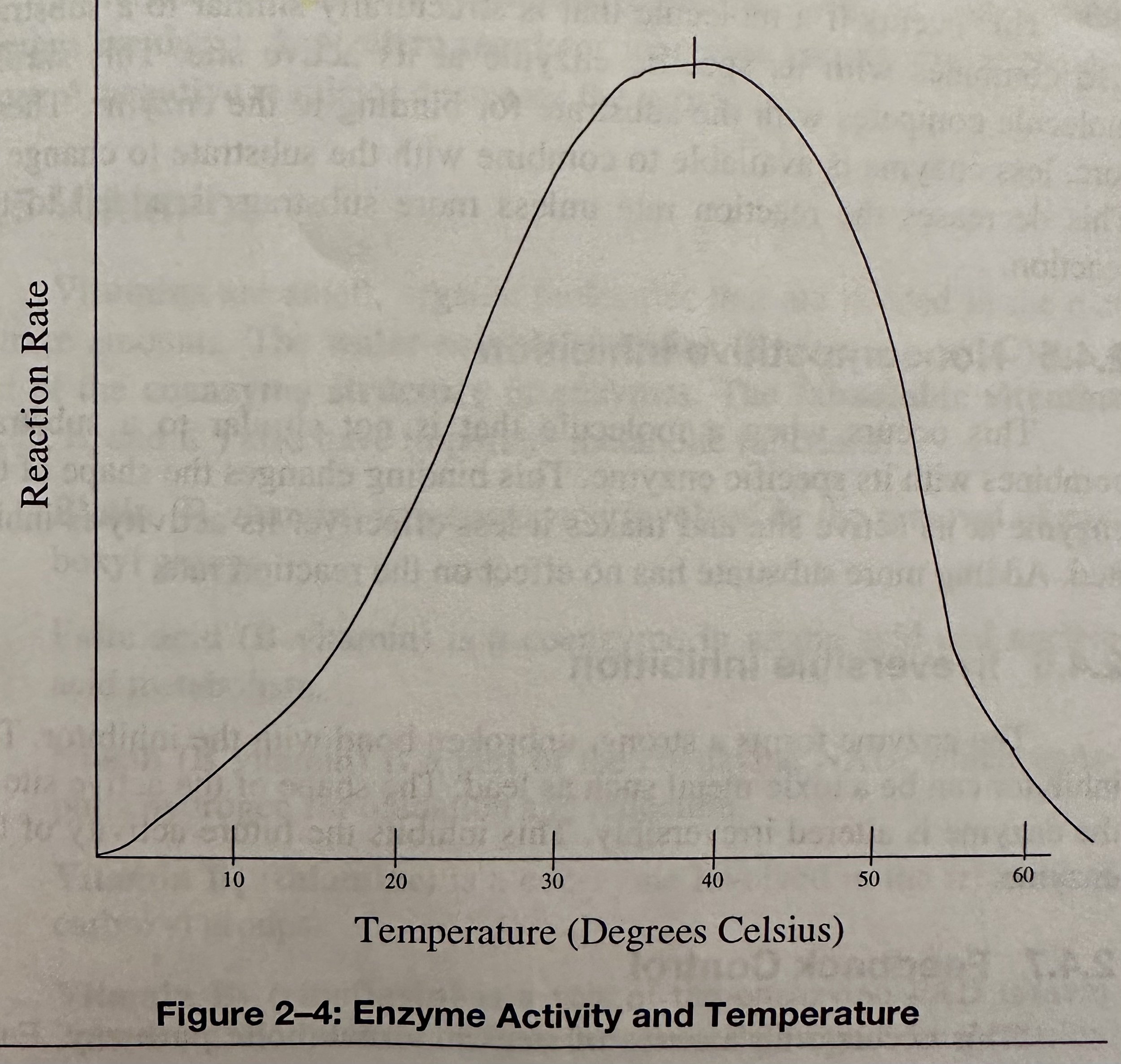
Enzymatic reactions have an optimum temperature. As the T increase, the reaction rate increases to about 37C. As the T continues to increase above 37C, the reaction rate gradually decreases.
_____________________________________________________________________________________
Steroids
Steroids: Named after the steroid cholesterol (one of the most common); a biologically active organic compound that is an important component of cell membranes that alter membrane fluidity and is a signaling molecule. The steroid core structure is typically composed of 17x C atoms, bonded in 4 “fused” rings: 3x 6-member cyclohexane rings and 1x 5-member cyclopentane ring. Steroids are not soluble in water.
Cholesterol: A common steroid that is part of the plasma membrane.
Low Density lipoprotein (LDL): Transport cholesterol from the liver to other cells of the body.
High Density lipoprotein (HDL): Remove cholesterol from dying cells throughout the body and return it to the liver. If excess cholesterol is not removed, it is deposited on the inside surfaces of arteries.
Hormones: A common steroid; chemical messengers transported by the blood; include the mineralcorticoids, the glucocorticoids, testosterone, estrogens, and progesterone. Hormones are secreted from an endocrine gland and released into the bloodstream, traveling to target tissues, where it has an effect. There are three general types of hormones: polypeptides (i.e. insulin), steroids (i.e. aldosterone), and amino acids products (i.e. epinephrine).
Ionic Balance: Many hormones regulate the concentration of different ions in the ECF. Aldosterone, secreted by the adrenal cortex, signals the kidneys to reabsorb Na. Thyrocalcitonin, secreted by the thyroid gland, lowers the concentration of Ca in the blood. PTH, from the parathyroid glands, increases Ca in the blood.
Mineralcorticoids: A hormone that regulates the concentration of minerals (ions) in the body.
Aldosterone: A hormone that signals the kidneys to reabsorb Na, preventing its elimination from the body when it is needed for various functions.
Glucorticoids: A hormone that regulates glucose metabolism, increasing the concentration of this sugar in the blood.
Testosterone: An androgen, the male sex hormone; it stimulates the development of male reproductive characteristics and other male characteristics.
Anabolic steroids: Very similar to testosterone; they can increase the mass of skeletal muscles in the body.
Estrogens: Include estradiol, female sex hormones. They stimulate the development of many female reproductive characteristics. During the menstrual cycle, these hormones stimulate the development and thickening of the endometrium, the inner lining of the uterus.
Progesterone: A female sex hormone; stimulates the development of the endometrium. Its concentration and action are high after ovulation. This prepares the endometrium for implantation of the embryo if fertilization occurs.
Insulin: A hormone that decreases the concentration of glucose in the blood and stimulates the production of glycogen from glucose. It also stimulates the production of lipids from glucose after the storage capacity for carbs has been fulfilled.
_____________________________________________________________________________________
Vitamins
Vitamins: Small, organic molecules that are needed in the diet in trace amounts. The water soluble (B and C) and the Fat Soluble (A, D, E, K).
Biotin (B Vitamin): A coenzyme involved in the removal of carboxyl groups.
Folic Acid (B Vitamin): A coenzyme in amino acid and nucleic acid metabolism.
Niacin (B Vitamin): A part of the coenzyme NAD, which transports H for oxidation and reduction.
B1 (Thiamine): A coenzyme involved in the removal of carboxyl groups.
B2 (Riboflavin): A part of the coenzyme FAD (Flavin Adenine Dinucleotide), which transports H for Oxidation and Reduction.
B6 (Pyridoxine): A coenzyme involved in the metabolism of nucleic acids.
C: Transports H ions and is an antioxidant.
A: A component in making a visual pigment in the rods of the retina of the eye, which function in dim light.
D: Needed for retention of Ca and P in the body. These minerals are needed for bone development.
E: Preserves Vitamin A and fatty acids. It is an antioxidant.
K: Needed to make proteins for the clotting of blood.
_____________________________________________________________________________________
Photosynthesis
Photosynthesis: The conversion of sunlight, water, and CO2 into Glucose.
Light Dependent Phase: A phase of photosynthesis in which the pigment chlorophyll traps light energy. This excites e- in the chlorophyll molecules. These e- escape and are transferred through a series of acceptors. The three main outcomes of the light dependent reactions are:
Production of ATP as the excited e- are passed through the acceptors.
Production of O as water undergoes photolysis, a breakdown in the presence of light. From this breakdown, O is made.
Production of NADPH as some e- are passed onto NADP combine with a proton to form NADPH. It enters the light-independent phase.
Light Independent Phase: A phase of photosynthesis; NADPH carries H to CO2. Through a series of reactions, the H’s, plus the C and O from CO2, make PGAL. PGAL is a 3 C compound. Every two molecules of PGAL are converted into one molecule of glucose.
_____________________________________________________________________________________
Nucleic Acids
Nucleic Acids: Long molecules consisting of bonded subunits called nucleotides. There are two important nucleic acids in biology- DNA and RNA. DNA directs the synthesis of RNA which makes proteins. On a chemical level, protein diversity accounts for the variety of life forms. Each species contains some proteins that make it unique.
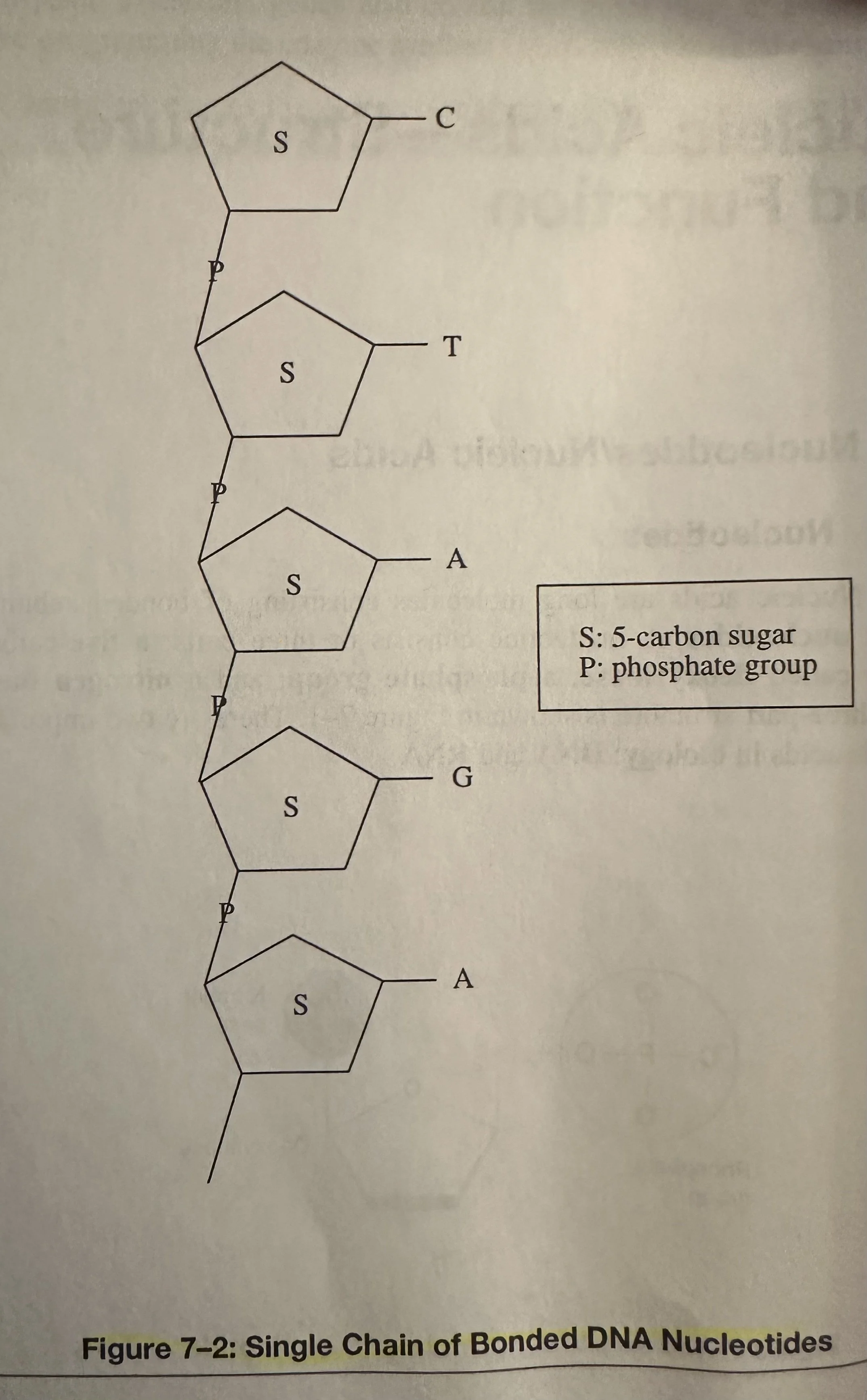
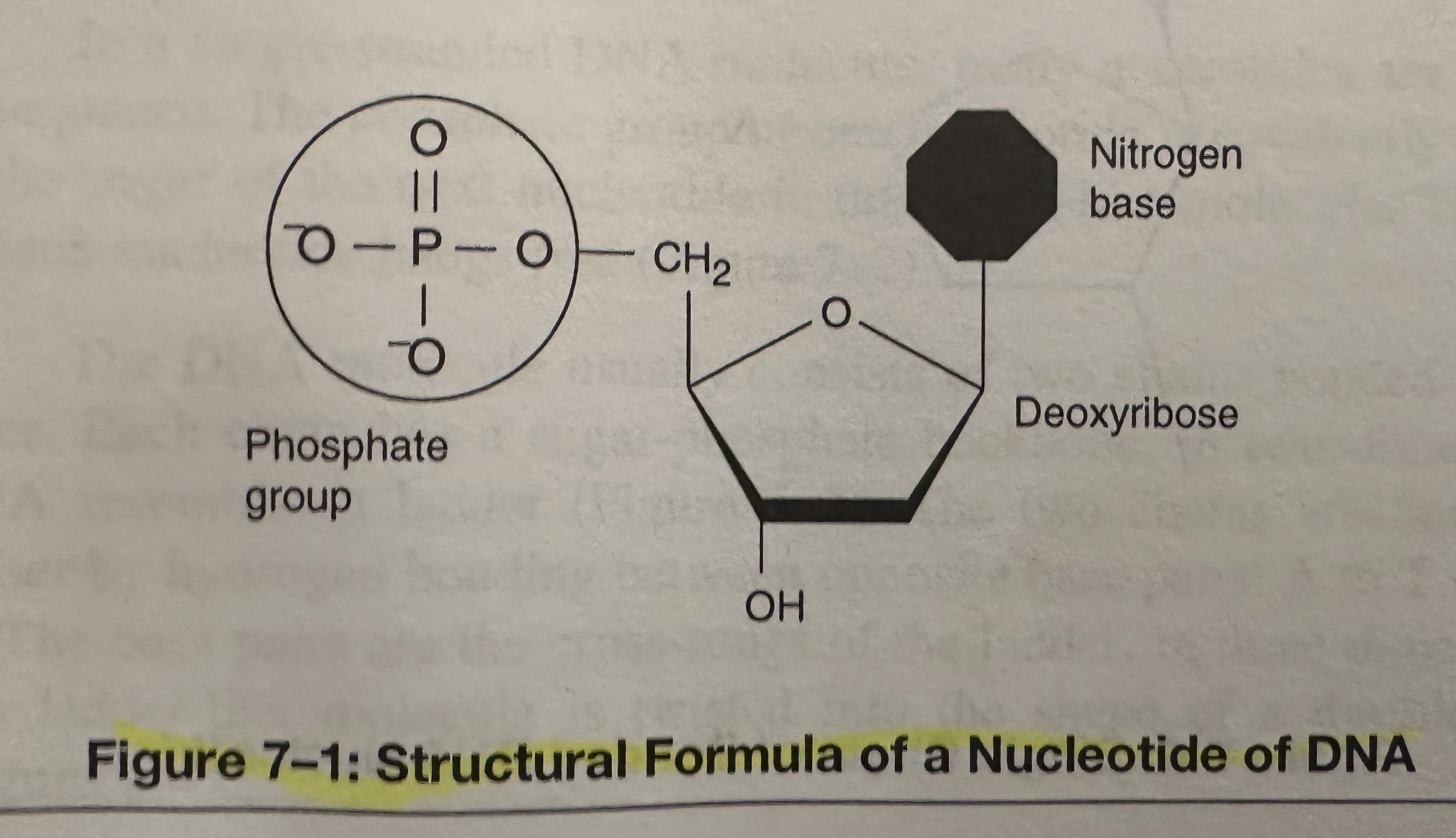
Nucleotides: Consists of three parts; a 5-C sugar called deoxyribose; a phosphate group; and a N base.
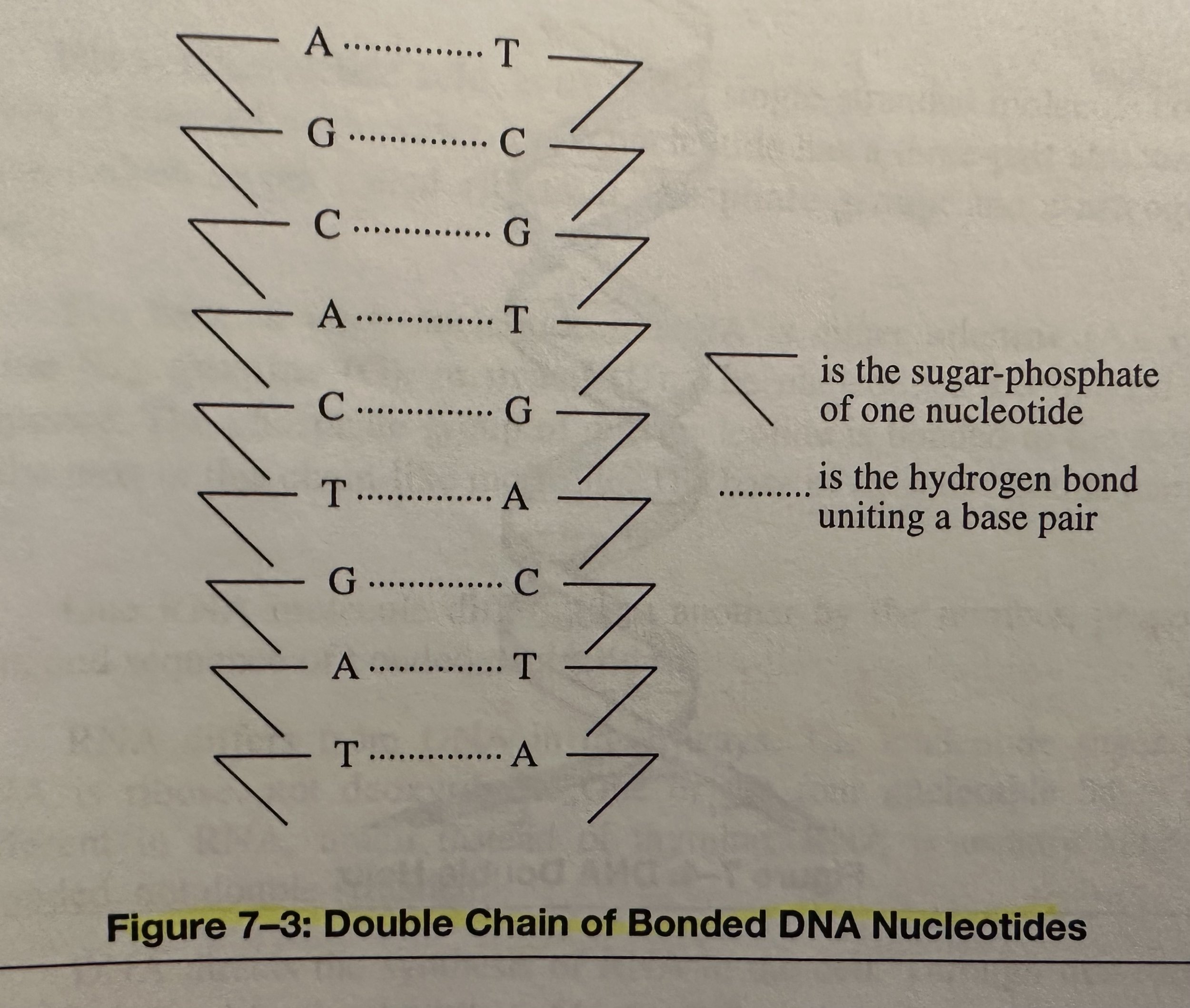
Deoxyribonucleic Acid (DNA): A nucleic acid that encodes the genetic information for the development of an organism’s traits, such as eye color and blood type; consists of two long chains of bonded nucleotides. The base of each nucleotide is either Adenine (A), Cytosine (C), Guanine (G), or thymine (T). DNA composes the genes, the units of heredity, on the chromosomes of the cell.
In a single-stranded DNA molecule, the phosphate group of one nucleotide is covalently bonded to the sugar of the next nucleotide. The base of each nucleotide hangs free.
Two chains are linked together by H bonding between opposite base pairs: A to T or G to C.
RNA: Usually a single-stranded molecule consisting of bonded nucleotides. Each nucleotide has a three-part structure: a 5-C sugar called ribose; a phosphate group; and a N base.
The base of each nucleotide in RNA is either adenine (A), cytosine (C), Guanine (G), or Uracil (U).
RNA differs from DNA in three ways:
The nucleotide sugar in RNA is ribose, not deoxyribose.
One of the four nucleotide bases is Uracil, not thiamine.
RNA is usually single stranded, not double stranded.
Messenger RNA (mRNA): Comprised of chromosomal DNA in the nucleus. It leaves the nucleus and enters the cytoplasm. At the ribosome it provides the genetic instructions to make proteins.
Transfer RNA (tRNA): Transcribed in the nucleus and enters the cytoplasm for protein synthesis. A tRNA molecules carries an amino acid.
Ribosomal RNA: Transcribed and becomes part of the structure of the ribosome.
_____________________________________________________________________________________
Replication, Transcription, Translation
DNA Replication: DNA can duplicate its structure through a process called replication. DNA makes RNA through a process called transcription. Through translation, RNA controls the synthesis of proteins.
Replication: The duplication of DNA. When a DNA double helix replicates, it produces two double molecules, each with the same number, proportion, and sequence of base pairs. The cell usually maintains a sufficient pool of four nucleotides to make new DNA chains. After the nucleotides with bases in each new strand are ordered, they bond to each other. An enzyme, DNA polymerase, controls this bonding
Transcription: The process of DNA making RNA; begins when the H bonds break in DNA and the two chains of the double helix separate. One of the free chains serves as a template to synthesize a strand of RNA. After the DNA orders the bases in the RNA, they bond to each other. An enzyme, RNA polymerase, controls this bonding.
Translation: Occurs at the ribosome; the synthesis of a polypeptide (a protein) from mRNA. The bases on the mRNA are decoded in groups of three- base triplets called codons. The cell maintains a pool of amino acids for protein synthesis. Each amino acid is recognized by a specific tRNA molecule, which brings its amino acid to the ribosome. A base triplet on the tRNA, the anticodon, is attracted to a codon of the mRNA. Translation occurs in three steps:
Initiation: The first codon and anticodon meet at the ribosome. Often, the start codon is AUG.
Elongation: The ribosome moves along the mRNA codon by codon, a process called translocation. As each codon is translate at the ribosome, another amino acid is properly placed for the polypeptide by its tRNA and anticodon. As more codons are translated, the length of the ordered amino acids increases.
As the amino acids are ordered, other enzymes form peptide bonds to connect them and form a polypeptide. Additional reactions assembly the polypeptides into an entire protein.
Termination: The synthesis is completed by a stop codon: UAA, UGA, or UAG. An enzyme releases the polypeptide from the ribosome.
_____________________________________________________________________________________
Genetics
Genetics: The study of heredity.
Heredity: The relationship between genes and the traits they control among parents and their offspring. Gene: The DNA that codes for the production of a polypeptide.
Genetic Code: A language of 64 codons that specifies the amino acids in polypeptides. 4 different bases, read in groups of three with repetition, yield 64 combinations.
Point Mutation: A change in one base of the DNA in a gene. A point mutation can result from an error when DNA is replicating.
Genetic Map: Identifies the genes on a chromosome.
Mutagens: Increase the probability for a mutation during replication.
_____________________________________________________________________________________
Cellular Fluids
The water in the human body is distributed through two regions, the ECF and ICF.
Extracellular Fluid (ECF): Water in the human body found outside the cells; ~2/3 of water in the body. ECF transports glucose, regulates glucose concentration, pH, and internal body temperature.
Intracellular Fluid (ICF): Water in the human body found inside the cells; ~1/3 of water in the body.
Fluid Balance: The hypothalamus of the brain monitors the osmolarity (solute concentration) of the blood. If this solute concentration increases with a water shortage, the hypothalamus signals the posterior pituitary gland to secrete ADH, the antidiuretic hormone. This hormone signals the kidney to reabsorb water.
_____________________________________________________________________________________
Blood
Blood Plasma: Comprises 55% of the blood and is ~90% H2O. O, CO2, and ions are dissolved in the plasma.
Albumins: Proteins that transport substances and contribute to osmotic balance in the plasma.
Globulins (Immunoglobulins): Protect the body against antigens (foreign substances) and invading cells.
Fibrinogen: Forms fibrin, a substance that traps cells when the blood clots.
Blood Cells
Erythrocytes: RBC’s that transport O and CO2.
Leucocytes: WBCs; part of the immune systems and provide a line of defense against foreign substances and invading cells.
Thrombocytes: Platelets; initiate the process of blood clotting.
Blood Clotting: Platelets of the blood swarm to a broken blood vessel, releasing a group of clotting factors that react in series. The last two substances to react in this series are plasma proteins produced by the liver, prothrombin and fibrinogen. Prothrombin reacts to form thrombin. Thrombin stimulates the conversion of fibrinogen into fibrin. Fibrin traps blood cells, forming a clot that plugs the broken vessel.
Blood Function: At the capillaries, substances leave the blood and enter the cells, meeting their needs of metabolism.
Blood Sugar: The concentration of blood sugar in humans normally ranges from 80-120 mg per 100ml. The level of glucose in the blood is controlled by at least 2 hormones secreted from the pancreas. If glucose increases in the plasma after a meal, the secretion of insulin removes the extra sugar. This returns the glucose to a normal concentration. The glucose is taken up by the liver and stored as glycogen. If the glucose level is low, such as during fasting, glucagon stimulates the production of glucose. Its release into the blood returns blood sugar to a normal level.
Insulin: Produced by the beta cells of the pancreas, lowers the level of glucose.
Glucagon: Produced by the alpha cells, increases the level of glucose.
Acids: Compounds that disassociate in water and release H ions. I.e., when HCL is dissolved in water, it dissociates to produce H+, H ions. These free ions are the source of acidity.
Buffers: Molecules that react against the acid or basic conditions of the blood, returning pH to normal. I.e, during acidosis, H ions accumulate in the blood. The buffer NaCO3 reacts with these ions, removing them and the source of acidity.
_____________________________________________________________________________________
Diseases
Many diseases are the results of the lack of proper nutrients or the body’s inability to correctly convert these nutrients.
Kwashiorkor: Develops in children from a severe deficiency of protein in the diet that results in extremity swelling.
Phenylketonuria (PKU): A metabolic disease resulting from the inability to chemically change phenylalanine. This is due to the absence of the enzyme needed to change it to tyrosine. If phenylalanine remains in the diet, it will increase in the blood. Its high concentration can cause mental retardation in newborn infants.
_____________________________________________________________________________________
Terminology
Active Transport: The movement of molecules from a region of lower concentration to a region of higher concentration (requires energy).
Aerobic Pathways: The Krebs cycle and electron transport chain are aerobic pathways; dependent on the presence of O.
C6H12O6 + 6O2 = 6CO2 + 6H2O + 36 ATPs.
Denaturation: The breaking of many of the weak linkages, or bonds, within a protein molecule that are responsible for the highly ordered structure of the protein in its natural state. Denatured proteins have a looser, more random structure; most are insoluble. Denaturation occurs through various means including hating, treatment with alkali, acid, urea, detergents, or shaking.
Diffusion: The movement of molecules from a region of higher concentration to a region of lower concentration.
Osmosis: The movement of water through a membrane by diffusion.
Digestion: The chemical digestion of carbs, lipids, and proteins is completed in the small intestine.
Histamine: Dilates blood vessels and increases blood flow in response to inflammation. The blood brings chemicals to repair damaged areas and cells to carry out phagocytes.
Homeostasis: The maintenance of a constant internal environment.
Kinetic Energy: The energy of motion.
Metabolism: The sum of all chemical reactions that occur in the organism.
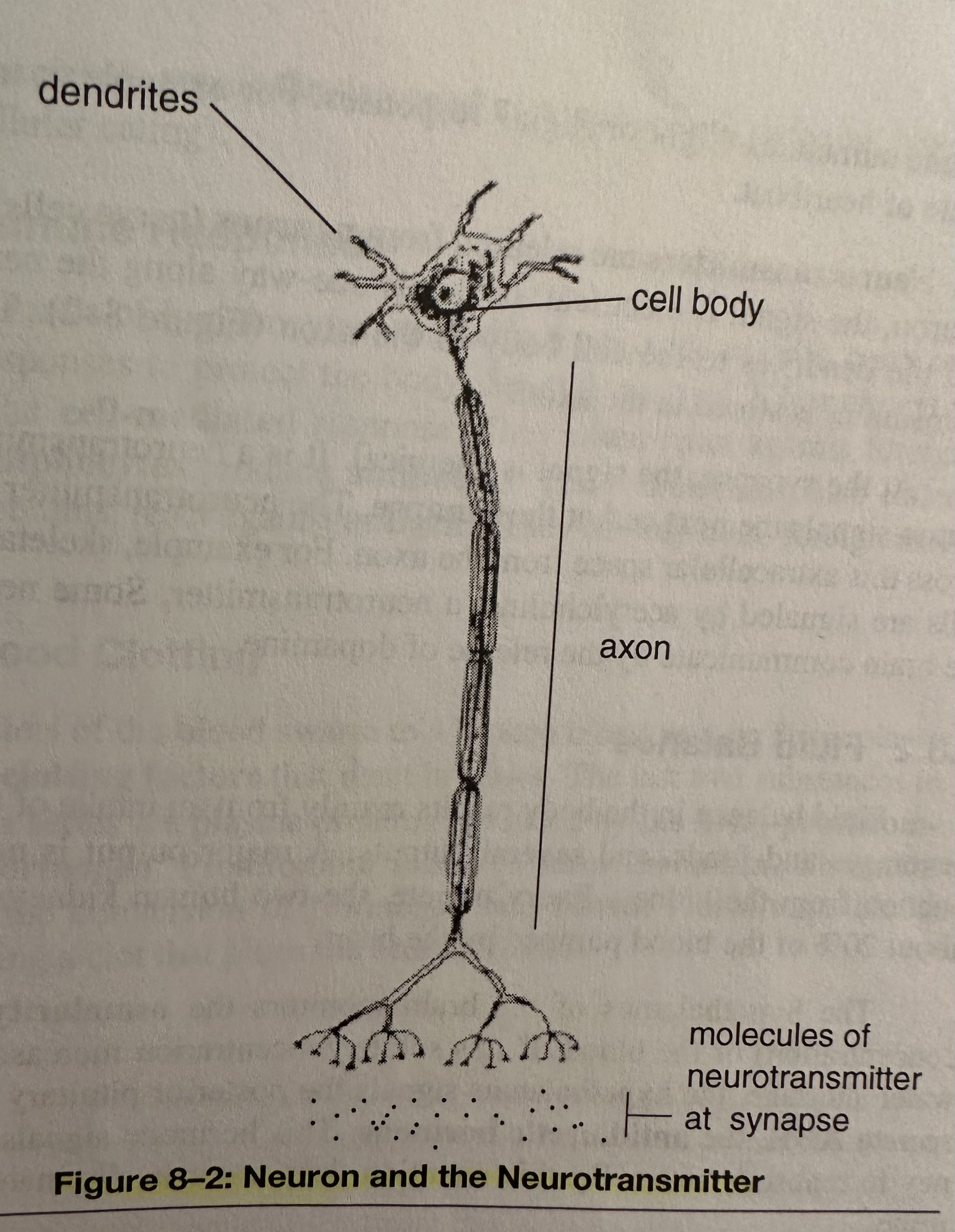
Neurotransmitters: A messenger molecule that is stored in the axon and released from neurons (nerve cells).
Permeability: The ability of a substance to pass through a membrane.
Polymer: A large molecule consisting of smaller molecules that are bonded together.
Functional Groups: Determines the shape and properties of polymers.
Salts: Compounds consisting of positive and negative ions (eg NaCl).
Structural Isomers: Molecules with the same molecular formula but different structural formulas.
_____________________________________________________________________________________