Six Easy Pieces by Feynman
Ref: Richard Feynman (1963). Six Easy Pieces. Basic Books.
___________________________________________________________________________
Summary
We have chosen the six easiest chapters from Feynman’s celebrated and landmark text, The Feynman Lectures on Physics (originally published in 1963), which remains his most famous publication.
Imagine that the complicated array of moving things (in nature) which constitutes “the world” is something like a great chess game being played by the gods, and we are observers of the game. We do not know what the rules of the game are; all we are allowed to do is to watch the playing. Of course, if we watch long enough, we may eventually catch on to a few of the rules. The rules of the game are what we mean by fundamental physics. Even if we knew every rule, however, we might not be able to understand why a particular move is made in the game, merely because it is too complicated and our minds are limited.
Atomic Hypothesis: Everything is made of atoms; everything that living things do can be understood in terms of the jigglings and wigglings of atoms.
___________________________________________________________________________
1 Atoms in Motion
Atomic Hypothesis: Everything is made of atoms.
If an apple is magnified to the size of the earth, then the atoms in the apple are ~ the size of the original apple.
Mass is found to increase with velocity, but appreciable increases require velocities near that of light.
When we compress a gas slowly, the temperature of the gas increases. So, under slow compression, a gas will increase in temperature, and under slow expansion it will decrease in temperature.
As we increase the temperature, atoms vibrate with greater and greater amplitude, until they shake themselves out of place. We call this melting. As we decrease the temperature, the vibration decreases and decreases until, at absolute zero, there is a minimum amount of vibration that the atoms can have, but not zero. This minimum amount of motion that atoms can have is not enough to melt a substance, with one exception: He. He merely decreases the atomic motions as much as it can, but even at absolute zero there is still enough motion to keep it from freezing.
The jiggling motion is what we represent as heat: when we increase the temperature, we increase the motion. If we heat the water, the jiggling increases and the volume between the atoms increases, and if the heating continues there comes a time when the pull between the molecules is not enough to hold them together and they do fly apart and become separated from one another. Of course, this is how we manufacture steam out of water- by increasing the temperature; the particles fly apart because of the increased motion.
If we take the top of a vessel off and blow the moist air away, replacing it with dry air, then the number of molecules leaving is just the same as it was before, because this depends on the jiggling of the water, but the number coming back is greatly reduced because there are so many fewer water molecules above the water. Therefore, there are more going out than coming in, and the water evaporates. Hence, if you wish to evaporate water turn on the fan!
In a salt crystal we find Cl ions (Cl atoms with an extra electron) and Na ions (sodium atoms with one electron missing). The ions all stick together by electrical attraction in the solid salt, but when we put them in the water we find, because of the attractions of the negative O and positive H for the ions, that some of the ions jiggle loose…If there is almost no salt in the water, more atoms leave than return, and the salt dissolves. If, on the other hand, there are too many “salt atoms,” more return than leave, and the salt is crystallizing.
C attracts O much more than O attracts O or C attracts C. Therefore, in this process the O may arrive with only a little energy, but the O and C will snap together with a tremendous vengeance and commotion, and everything near them will pick up the energy. A large amount of motion energy, kinetic energy, is thus generated. This of course is burning; we are getting heat from the combination of O and C. The heat is ordinarily in the form of the molecular motion of the hot gas, but in certain circumstances it can be so enormous that it generates light. That is how one gets flames.
CO2 molecules are straight and symmetrical: O—C—O.
___________________________________________________________________________
2 Basic Physics
At first the phenomena of nature were roughly divided into classes, like heat, electricity, mechanics, magnetism, properties of substances, chemical phenomena, light or optics, x-rays, nuclear physics, gravitation, meson phenomena, etc. However, the aim is to see complete nature as different aspects of one set of phenomena. That is the problem in basic theoretical physics today—to find the laws behind experiment; to amalgamate these classes.
Pressure: The collision of atoms with walls or whatever.
Wind: The drift of atoms if they are all moving in one direction on the average.
Heat: The random internal motions of atoms; the more motion, the more heat the system contains.
Sound: Waves of atoms of excess density.
Magnetism: The influence of charged atoms in relative motion.
The chemical properties of a substance depend only on the number of electrons.
A more adequate representation of the situation is to say that the existence of the positive charge, in some sense, distorts, or creates a “condition” in space, so that when we put the negative charge in, it feels a force. This potentiality for producing a force is called an electric field. When we put an electron in an electric field, we say it is “pulled.” We then have two rules: (a) charges make a field, and (b) charges in fields have forces on them and move.
EM rays coming from nuclei are called gamma rays, while those of high energy from atoms are called x-rays, but at the same frequency they are indistinguishable physically.
An atom has a diameter of about 10-8 cm. The nucleus has a diameter of about 10-13 cm. If we had an atom and wished to see the nucleus, we would have to magnify it until the whole atom was the size of a large room, and then the nucleus would be a bare speck which you could just about make out with the eye, but very nearly all the weight of the atom is in that infinitesimal nucleus. What keeps the electrons from simply falling in? This principle: If they were in the nucleus, we would know their position precisely, and the uncertainty principle would then require that they have a very large (but uncertain) momentum, i.e., a very large KE. With this energy they would break away from the nucleus. They make a compromise: they leave themselves a little room for this uncertainty and then jiggle with a certain amount of minimum motion in accordance with this rule.
Things which we used to consider as waves also behave like particles, and particles behave like waves; in fact everything behaves the same way. There is no distinction between a wave and a particle. So quantum mechanics unifies the idea of the field and its waves, and the particles, all into one. Now it is true that when the frequency is low, the field aspect of the phenomenon is more evident, or more useful as an approximate description in terms of everyday experiences. But as the frequency increases, the particle aspects of the phenomenon become more evident with the equipment with which we usually make the measurements. In fact, although we mentioned many frequencies, no phenomenon directly involving a frequency has yet been detected above approximately 1012 cycles per second. We only deduce the higher frequencies from the energy of the particles, by a rule which assumes that the particle-wave idea of quantum mechanics is valid.
Quantum Electrodynamics (QED): The fundamental theory of the interaction of light and matter, electrons and photons, or electric field and charges. QED governs the basic rules for all ordinary phenomena except for gravitation and nuclear processes. QED predicts a lot of new things. In the first place, it tells the properties of very high-energy photons, gamma rays, etc. It predicted another very remarkable thing: besides the electron, there should be another particle of the same mass, but of opposite charge, called a positron, and these two, coming together, could annihilate each other with the emission of light or gamma rays.
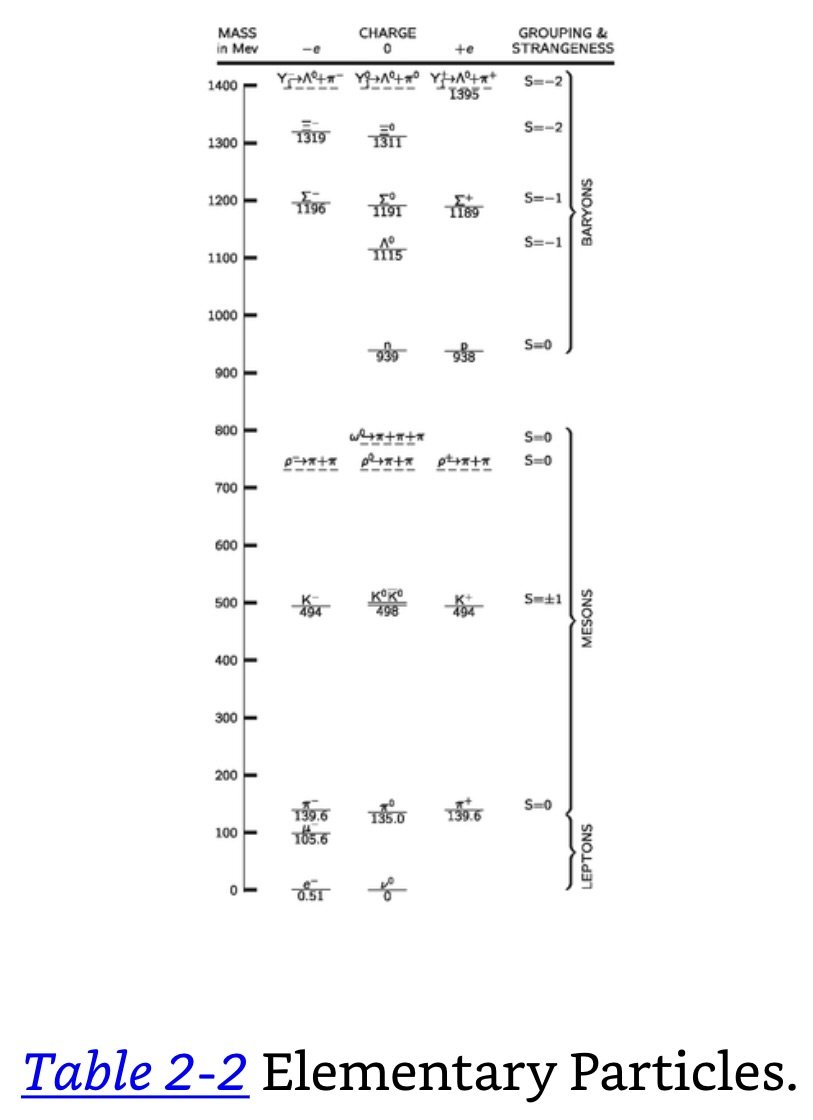
Today we have ~30 particles. All are unstable except the electron, neutrino, photon, graviton, and proton.
Photon: Zero-mass, zero-charge particles that are always moving at c.
Graviton: Zero-mass, zero-charge particles.
Baryons: All particles together with neutrons and protons.
Leptons: Particles including the electron (.510 MeV), μ-meson, the muon (105 MeV).
Neutrino: A neutrally charged particle with zero mass. There are two different kinds of neutrinos, one related to electrons and the other related to muons.
MeV: 1.782 × 10-27 gram.
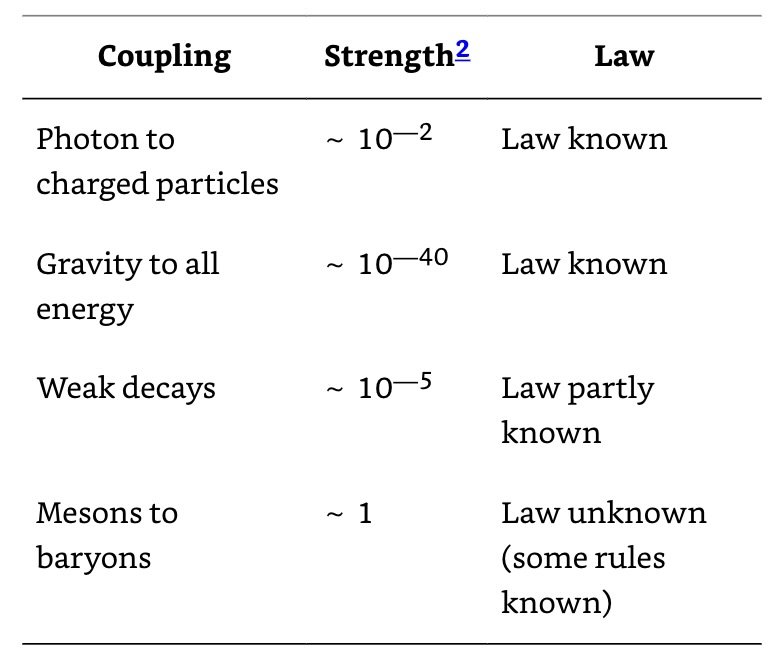
There seem to be just four kinds of interaction between particles which, in the order of decreasing strength, are the nuclear force, electrical interactions, the beta-decay interaction (which causes the neutron to disintegrate into proton, electron, and neutrino), and gravity.
___________________________________________________________________________
3 The Relation of Physics to Other Sciences
Physical Chemistry: The study of the rates at which reactions occur and what is happening in detail.
Organic Chemistry: The chemistry of the substances which are associated with living things.
Nerves are very fine tubes with a complex wall which is very thin; through this wall the cell pumps ions, so that there are positive ions on the outside and negative ions on the inside, like a capacitor. If this membrane “discharges” in one place, i.e., if some of the ions were able to move through one place, so that the electric voltage is reduced there, that electrical influence makes itself felt on the ions in the neighborhood, and it affects the membrane in such a way that it lets the ions through at neighboring points also. This in turn affects it farther along, etc., and so there is a wave of “penetrability” of the membrane which runs down the fiber when it is “excited” at one end by stepping on the sharp stone. This wave is somewhat analogous to a long sequence of vertical dominoes; if the end one is pushed over, that one pushes the next, etc. Of course, this will transmit only one message unless the dominoes are set up again; and similarly in the nerve cell, there are processes which pump the ions slowly out again, to get the nerve ready for the next impulse…What happens at the end of the nerve? There the nerve branches out into fine little things, connected to a structure near a muscle, called an endplate. For reasons which are not exactly understood, when the impulse reaches the end of the nerve, little packets of a chemical called acetylcholine are shot off (five or ten molecules at a time) and they affect the muscle fiber and make it contract.
Enzymes: Comprised of proteins; enzymes are very big and complicated, and each one is different, each being built to control a certain special reaction. Enzymes themselves are not involved in reactions directly. They do not change; they merely let an atom go from one place to another. Having done so, the enzyme is ready to do it to the next molecule, like a machine in a factory.
The transformation of Guanosine-di-phosphate to Guanosine-tri-phosphate (GDP to GTP); because the one substance has much more energy in it than the other. Just as there is a “box” in certain enzymes for carrying H atoms around, there are special energy-carrying “boxes” which involve the triphosphate group. So, GTP has more energy than GDP and if the cycle is going one way, we are producing molecules which have extra energy and which can go drive some other cycle which requires energy.
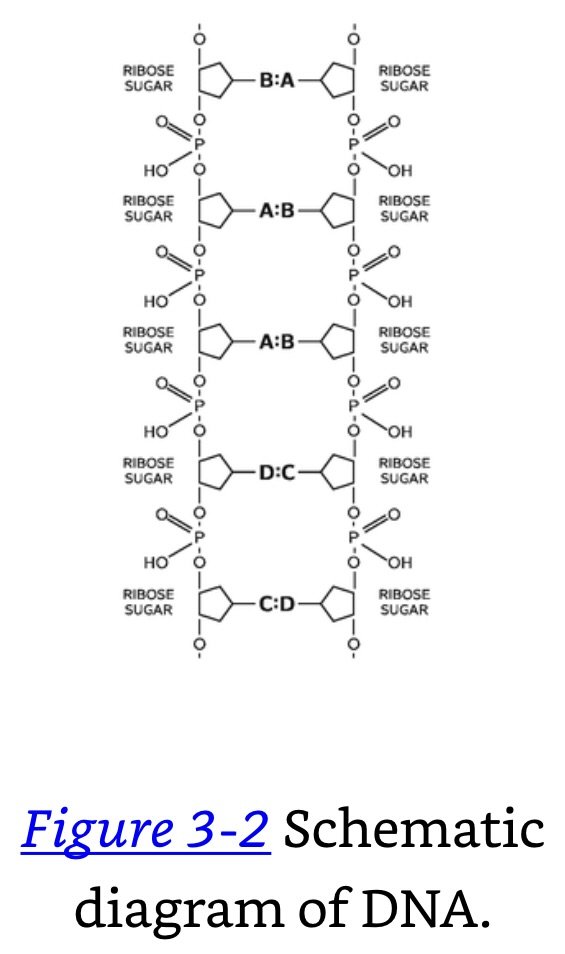
Proteins: A series, or chain, of different amino acids. There are 20 amino acids, and they all can combine with each other to form chains in which the backbone is CO-NH, etc. Proteins are made in the ribosomes.
How does the order of the A, B, C, D units determine the arrangement of the amino acids in the protein? This is the central unsolved problem in biology today.
RNA: A small molecular piece carrying information that comes off the DNA and carries a message as to what kind of protein to make goes over to the ribosome. When it gets there, protein is synthesized at the ribosome.
___________________________________________________________________________
4 Conservation of Energy
Conservation of Energy: There is a certain quantity, which we call energy, that does not change in the manifold changes which nature undergoes. There is a numerical quantity which does not change when something happens. It is not a description of a mechanism, or anything concrete; it is just a strange fact that we can calculate some number and when we finish watching nature go through her tricks and calculate the number again, it is the same (governs all known natural phenomenon without exception).
Forms of energy- gravitational, kinetic, heat, elastic, electrical, chemical, radiant, nuclear, mass.
“It is important to realize that in physics today, we have no knowledge of what energy is (Feynman).”
Potential Energy: Energy which has to do with location relative to something else.
Change in Energy = F times the d that the F is pushed.
Mass Energy: An object has energy from its sheer existence. If two particles come together and disappear, radiant energy will be liberated, in a definite amount, and the amount can be calculated per E = mc2.
Conservation of Momentum: Momentum = mass of an object multiplied by its velocity; within some problem domain, the amount of momentum remains constant; momentum is neither created nor destroyed, but only changes through the action of forces per Newton’s Laws (NASA).
Conservation of Mass: Mass = volume of an object multiplied by the density of the object; Within some problem domain, the amount of mass remains constant- mass is neither created nor destroyed (NASA).
Conservation of Baryons: In any reaction in nature, if we count how many baryons are coming into a process, the number of baryons which come out will be exactly the same.
Conservation of Leptons: In any reaction in nature, if we count how many leptons (electrons, mu meson, neutrino) are coming into a process, the number of leptons which come out will be exactly the same.
Conservation of Charge: In any reaction in nature, the number of positive, minus, and electrical charges is never changed.
Laws of Thermodynamics: The laws which govern how much energy is available; involves entropy for irreversible thermodynamic processes.
___________________________________________________________________________
5 The Theory of Gravitation
Law of Gravitation: Every object in the universe attracts every other object with a force which for any two bodies is proportional to the mass of each and varies inversely as the square of the distance between them.
An object responds to a force by accelerating in the direction of the force by an amount that is inversely proportional to the mass of the object.
As far as we know, gravity seems to go out forever inversely as the square of the distance.
Kepler’s Laws
Each planet moves around the sun in an ellipse, with the sun at one focus.
The radius vector from the sun to the planet sweeps out equal areas in equal intervals of time.
The squares of the periods of any two planets are proportional to the cubes of the semimajor axes of their respective orbits: T ∝ a3/2.
Principle of Inertia: If something is moving, with nothing touching it and completely undisturbed, it will go on forever, coasting at a uniform speed in a straight line.
Newton modified this idea, saying that the only way to change the motion of a body is to use force. If the body speeds up, a force has been applied in the direction of motion. On the other hand, if its motion is changed to a new direction, a force has been applied sideways. Newton thus added the idea that a force is needed to change the speed or the direction of motion of a body.
If a stone is attached to a string and is whirling around in a circle, it takes a force to keep it in the circle. We have to pull on the string. In fact, the law is that the acceleration produced by the force is inversely proportional to the mass, or the force is proportional to the mass times the acceleration. The more massive a thing is, the stronger the force required to produce a given acceleration…The brilliant idea resulting from these considerations is that no tangential force is needed to keep a planet in its orbit (the angels do not have to fly tangentially) because the planet would coast in that direction anyway. If there were nothing at all to disturb it, the planet would go off in a straight line. But the actual motion deviates from the line on which the body would have gone if there were no force, the deviation being essentially at right angles to the motion, not in the direction of the motion. In other words, because of the principle of inertia, the force needed to control the motion of a planet around the sun is not a force around the sun but toward the sun.
It was already known that the planet Jupiter had moons going around it as the moon of the earth goes around the earth, and Newton felt certain that each planet held its moons with a force. He already knew of the force holding us on the earth, so he proposed that this was a universal force- that everything pulls everything else.
Cavendish first demonstrated the direct force between two large, fixed balls of Pb and two smaller balls of Pb on the ends of an arm supported by a very fine fiber, called a torsion fiber. By measuring how much the fiber gets twisted, one can measure the strength of the force, verify that it is inversely proportional to the square of the distance, and determine how strong it is. Thus, one may accurately determine the coefficient G in the gravitational formula.
The force of electricity between two charged objects looks just like the law of gravitation: the force of electricity is a constant, with a minus sign, times the product of the charges, and varies inversely as the square of the distance.
If we take, in some natural units, the repulsion of two electrons (nature’s universal charge) due to electricity, and the attraction of two electrons due to their masses, we can measure the ratio of electrical repulsion to the gravitational attraction. The ratio is independent of the distance and is a fundamental constant of nature…Consider the time it takes light to go across a proton, 10-24 second. If we compare this time with the age of the universe, 2 × 1010 years, the answer is 10-42. It has about the same number of zeros going off it, so it has been proposed that the gravitational constant is related to the age of the universe.
Newton’s law of gravitation was modified by Einstein to take into account the theory of relativity. According to Newton, the gravitational effect is instantaneous, that is, if we were to move a mass, we would at once feel a new force because of the new position of that mass; by such means we could send signals at infinite speed. Einstein advanced arguments which suggest that we cannot send signals faster than the speed of light, so the law of gravitation must be wrong. By correcting it to take the delays into account, we have a new law, called Einstein’s law of gravitation. One feature of this new law which is quite easy to understand is this: In the Einstein relativity theory, anything which has energy has mass- mass in the sense that it is attracted gravitationally. Even light, which has an energy, has a “mass.” When a light beam, which has energy in it, comes past the sun there is an attraction on it by the sun. Thus, the light does not go straight, but is deflected.
All mass is made of tiny particles and that there are several kinds of interactions, such as nuclear forces, etc. None of these nuclear or electrical forces has yet been found to explain gravitation. The quantum-mechanical aspects of nature have not yet been carried over to gravitation.
___________________________________________________________________________
6 Quantum Behavior
Quantum Mechanics: The description of the behavior of matter in all its details and, in particular, of the happenings on an atomic scale. Things on a very small scale behave like nothing that you have any direct experience about. They do not behave like waves, they do not behave like particles, they do not behave like clouds, or billiard balls, or weights on springs, or like anything that you have ever seen. The quantum behavior of atomic objects (electrons, protons, neutrons, photons, etc) is the same for all; they are all “particle waves,” or whatever you want to call them.
Electrons: Behave like light.
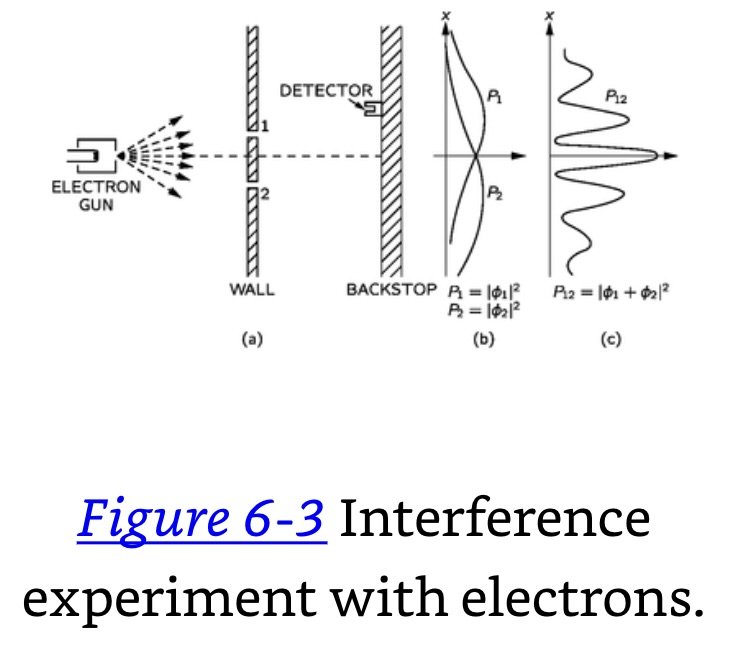
Electron- Double Slit- Backstop Experiment: Electrons are shot towards a surface with two openings. Any electrons that pass through the opening is counted on a backdrop.
The electrons arrive in lumps, like particles, and the probability of arrival of these lumps is distributed like the distribution of intensity of a wave. It is in this sense that an electron behaves “sometimes like a particle and sometimes like a wave.”
Electron- Double Slit- Backstop with Light Source Experiment: To our electron apparatus we add a very strong light source, placed behind the wall and between the two holes. We know that electric charges scatter light. So, when an electron passes, however it does pass, on its way to the detector, it will scatter some light to our eye, and we can see where the electron goes.
Here is what we see: every time that we hear a “click” from our electron detector (at the backstop), we also see a flash of light either near hole 1 or near hole 2, but never both at once.
It must be that the electrons are very delicate, and the light, when it scatters off the electrons, gives them a jolt that changes their motion. We know that the electric field of the light acting on a charge will exert a force on it. So perhaps we should expect the motion to be changed. Anyway, the light exerts a big influence on the electrons. By trying to “watch” the electrons we have changed their motions. That is, the jolt given to the electron when the photon is scattered by it is such as to change the electron’s motion enough so that if it might have gone to where P12 was at a maximum, it will instead land where P12 was a minimum; that is why we no longer see the wavy interference effects.
When we do not see the electron, no photon disturbs it, and when we do see it, a photon has disturbed it. There is always the same amount of disturbance because the light photons all produce the same-sized effects and the effect of the photons being scattered is enough to smear out any interference effect.
If we want to disturb the electrons only slightly, we should not have lowered the intensity of the light; we should have lowered its frequency (the same as increasing its wavelength).
It is impossible to arrange the light in such a way that one can tell which hole the electron went through, and at the same time not disturb the pattern.
We do not know how to predict what would happen in a given circumstance, and we believe now that it is impossible, that the only thing that can be predicted is the probability of different events.
A photon’s momentum is inversely proportional to its wavelength (p = h/λ).
Ideal Experiment: One in which all of the initial and final conditions of the experiment are completely specified.
Event: A specific set of initial and final conditions (i.e., an electron leaves an electron gun, arrives at the detector, and nothing else happens).
Event probability in an ideal experiment = the square of the absolute value of a complex number φ (probability amplitude). When an event can occur in several alternative ways, the φ for the event is the sum of the probability amplitudes for each way considered separately. There is interference. If an experiment is performed which is capable of determining whether one or another alternative is actually taken, the probability of the event is the sum of the probabilities for each alternative. The interference is lost.
Uncertainty Principle: It is impossible to design an apparatus to determine which hole the electron passes through, that will not at the same time disturb the electrons enough to destroy the interference pattern.
“If you make the measurement on any object, and you can determine the x-component of its momentum with an uncertainty Δp, you cannot, at the same time, know its x-position more accurately than Δx ≥ ħ/2Δp. The uncertainties in the position and momentum at any instant must have their product greater than half the reduced Planck constant”(Heisenberg’s Uncertainty Principle).
___________________________________________________________________________
Misc Quotes
“Each piece, or part, of the whole of nature is always merely an approximation to the complete truth, or the complete truth so far as we know it. In fact, everything we know is only some kind of approximation, because we know that we do not know all the laws as yet. Therefore, things must be learned only to be unlearned again or, more likely, to be corrected. The principle of science, the definition, almost, is the following: The test of all knowledge is experiment. Experiment is the sole judge of scientific “truth.”
“The power of instruction is seldom of much efficacy except in those happy dispositions where it is almost superfluous (Gibbon).”
“First figure out why you want the students to learn the subject and what you want them to know, and the method will result more or less by common sense (Feynman).”
“If, in some cataclysm, all of scientific knowledge were to be destroyed, and only one sentence passed on to the next generations of creatures, what statement would contain the most information in the fewest words? I believe it is the atomic hypothesis (or the atomic fact, or whatever you wish to call it) that all things are made of atoms- little particles that move around in perpetual motion, attracting each other when they are a little distance apart, but repelling upon being squeezed into one another.”
“Psychoanalysis is not a science: it is at best a medical process, and perhaps even more like witch-doctoring…Psychoanalysis has not been checked carefully by experiment, and there is no way to find a list of the number of cases in which it works, the number of cases in which it does not work, etc.”
“What strange array of chemicals are in the wine? How did they come to be? There are the ferments, the enzymes, the substrates, and the products. There in wine is found the great generalization: all life is fermentation.”
“Theoretical physicists are those who imagine, deduce, and guess at new laws, but do not experiment; and then there are experimental physicists who experiment, imagine, deduce, and guess.”
“Poets do not write to be understood (Feynman).”
“Only one part in 2B of solar energy falls on the earth.”
“It is better to perform some careful experiments than to carry on deep philosophical arguments (Feynman).”
___________________________________________________________________________
Terminology
Air: Consists of N, O, some H2O vapor, and lesser amounts of CO2, Ar, and other things.
Angstrom (Å): 10-8 cm. Atoms are ~1-2 Å in radius.
Brownian Motion: A perpetual jiggling of very tiny particles (colloids), the result of the bombardment of the atoms. This is called the Brownian motion.
Ideal Experiment: An experiment in which there are no uncertain external influences.
Scientific Method: Observation, reason, and experiment.
___________________________________________________________________________
Chronology
~1947: Discovery of the π-meson (‘pion’), required for nuclear forces (with the proton and neutron) (Six Easy Pieces by Feynman).
1900: German physicist Max Planck proposes that light and other EM radiation, hitherto regarded as waves, paradoxically behaved like tiny packets of energy, or “quanta,” when interacting with matter. These particular quanta became known as photons (Six Easy Pieces by Feynman).
1656: Scientists exploring the position and timing of Jupiter’s moons discern that light does not travel instantaneously (Six Easy Pieces by Feynman).
___________________________________________________________________________